Wilderness Medical Society Practice Guidelines for Treatment of Exercise-Associated Hyponatremia: 2014 Update

Brad L. Bennett, PhD; Tamara Hew-Butler, DPM, PhD; Martin D. Hoffman, MD; Ian R. Rogers, MD; Mitchell H. Rosner, MD
From the Military & Emergency Medicine Department, F. Edward Hebert School of Medicine, Uniformed Services University of the Health Sciences, Bethesda, MD (Dr Bennett); Oakland University, Rochester, MI (Dr Hew-Butler); the Department of Physical Medicine & Rehabilitation, Department of Veterans Affairs, Northern California Health Care System, and University of California Davis Medical Center, Sacramento, CA (Dr Hoffman); St. John of God Murdoch Hospital & University of Notre Dame, Murdoch, Western Australia (Dr Rogers); and the Division of Nephrology, University of Virginia, Charlottesville, VA (Dr Rosner).
Exercise-associated hyponatremia (EAH) is defined by a serum or plasma sodium concentration below the normal reference range of 135 mmol/L that occurs during or up to 24 hours after prolonged physical activity. It is reported to occur in individual physical activities or during organized endurance events conducted in austere environments in which medical care is limited and often not available, and patient evacuation to definitive care is often greatly delayed. Rapid recognition and appropriate treatment are essential in the severe form to ensure a positive outcome. Failure in this regard is a recognized cause of event-related fatality. In an effort to produce best practice guidelines for EAH in the austere environment, the Wilderness Medical Society convened an expert panel. The panel was charged with the development of evidence-based guidelines for management of EAH. Recommendations are made regarding the situations when sodium concentration can be assessed in the field and when these values are not known. These recommendations are graded on the basis of the quality of supporting evidence and balance between the benefits and risks/burdens for each parameter according to the methodology stipulated by the American College of Chest Physicians. This is an updated version of the original WMS Practice Guidelines for Treatment of Exercise-Associated Hyponatremia published in Wilderness & Environmental Medicine 2013;24(3):228–240.
Key words:hyponatremia, exercise-associated hyponatremia, arginine vasopressin, SIADH, exercise
Introduction
Nearly 3 decades after the first report of exercise-associated hyponatremia (EAH),1 great strides are taking place in an effort to prevent what is now recognized as a leading cause of preventable morbidity and mortality in endurance activities throughout the world. To date, review articles and international consensus statements have documented risk factors, pathophysiology, signs and symptoms, prevention, and patient management strategies.2–8 These reports have primarily focused on incidences of EAH in organized endurance events that are conducted in the front country where medical tents and local emergency medical services are available on site to assist these participants and to transport as needed to a local hospital for appropriate management. Beyond front country triathlons and marathons, many prolonged individual activities, ultramarathons, and multiple-day endurance events take place in the back-country. EAH has been documented in hikers, trekkers, climbers, and cold climate endurance athletes.9 –14 Furthermore, it is likely that many individuals with symptomatic or asymptomatic EAH go under reported in the literature.15 The lessons learned from current evidence- based EAH guidelines can be extended to those providing care in the backcountry in a limited-resource environment. It was the intent of this panel to develop evidence-based practice guidelines for EAH for use in austere environments, during transport by emergency medical services, and for immediate care by the receiving hospital.
This set of guidelines is an updated version of the original Wilderness Medical Society Practice Guidelines for Treatment of Exercise-Associated Hyponatremia published in Wilderness & Environmental Medicine 2013;24(3):228–240.
Methods
The expert panel was convened at the Wilderness Medical Society annual meeting in Whistler, British Columbia, Canada, July 2012. Members were selected on the basis of clinical interest or research experience. Relevant articles were identified by a search of MED- LINE as the primary database, US National Library of Medicine, National Institutes of Health (http://www.ncbi.nlm.nih.gov/pubmed/). Key search terms used were hyponatremia, exercise-associated hyponatremia, arginine vasopressin, syndrome of inappropriate antidiuretic hormone (SIADH), hyponatremic encephalopathy, and 3% hypertonic saline. Peer-reviewed studies related to EAH, including randomized controlled trials, observational studies, and case series, were reviewed, and the level of evidence supporting the conclusions was assessed. Abstract-only studies were not included. Conclusions from review articles were not considered in the formulation of recommendations but are cited below in an effort to provide context. When no relevant studies were identified, the panel recommendation was based on risk vs benefit perceptions derived from patient-care experience. The panel used a consensus approach to develop recommendations regarding management of EAH in the wilderness. These recommendations have been graded on the basis of clinical strength as outlined by the American College of Chest Physicians (ACCP; see the onlineSupplementary ACCP Table 1).16
Scope of the Problem
EAH is defined by a serum or plasma sodium concentration below the normal reference range of 135 mmol/L that occurs during or up to 24 hours after prolonged physical activity.7 The reported incidence of EAH varies widely, in part because the diagnosis is based solely on an abnormal biochemical result in an appropriate clinical setting. Many cases of EAH may be asymptomatic and are largely detected from blood samples taken from consenting athletes participating in research screening protocols, with reported incidence ranging from 0% to 51%. The highest reported incidence of“asymptomatic” hyponatremia has been noted in ultramarathon races covering 161 km (100 miles) in North America, in which the incidence of EAH has ranged between 30% and 51%.13,17–19
The incidence of asymptomatic EAH is greater than the incidence of “symptomatic” EAH, which refers to a biochemical diagnosis of EAH combined with clinical symptoms and signs. Severe EAH manifests as significant mental status changes resulting from cerebral edema (termed exercise-associated hyponatremic encephalopathy [EAHE]), at times associated with non-cardiogenic pulmonary edema.5,6Twelve confirmed deaths of public record have been directly attributed to complications associated with EAHE.20–24 The overall incidence of symptomatic EAH in all marathon participants is typically less than 1%,24,25 but the percentage of EAH seen in all symptomatic athletes seeking medical care has been reported to be as high as 23% in an Ironman Triathlon26 and 38% in runners participating in a marathon and ultramarathon in Asia.27 An increasing trend is that symptomatic EAH is now being reported in much shorter distance events, such as a half marathon28 and sprint triathlon taking approximately 90 minutes to complete.29
Symptomatic cases of EAH have been reported with increased frequency in both hikers and military infantry personnel. The reported incidence of hyponatremia in Grand Canyon hikers seeking medical care from exercise-associated collapse or exhaustion from May 31, 1993, through September 31, 1993, was 16% with an estimated incidence rate between 2.0 and 4.0 per 100,000 persons.9,30 Furthermore, suspected hyponatremia was found to account for 19% of nonfatal heat related incidents in Grand Canyon National Park from April through September during 2004 through 2009.31 US military services have reported an increased trend of EAH cases primarily in Marine Corps and Army infantry personnel of the past decade.32,33 However, new data (1999 to 2012) released in March 2013 shows an EAH incidence rate of 6.7 cases/100,000 person-years in US military services. These new data suggest that the annual incidence of EAH may have decreased by almost 50% from 2010 to 2012.34 It is important to note that one previously published paper entitled “Death by Water Intoxication” details 4 fatal cases of dilutional hyponatremia in military personnel.32 Whether or not all of these fatalities were primarily associated with EAH has been called into question. Thus, although it has been widely propagated that there have been 4 military deaths associated with EAH as the primary pathogenic mechanism, the number of EAH military deaths may be as low as 1 or 2. Nevertheless, modest hiking and marching activities in young and healthy individuals have led to documented EAHE morbidity and mortality.32,35
Pathogenesis of EAH
Two major pathologic mechanisms largely account for the development of EAH:
- excessive fluid intake, and
- impaired urinary water excretion, largely as a result of persistent secretion of arginine vasopressin (AVP), also referred to as antidiuretic hormone or ADH.4,5
EXCESSIVE FLUID INTAKE
Overhydration appears to be the primary risk factor for the development of EAH. This is reflected in the weight gains seen in the majority of, but not all, athletes who become symptomatic with EAH. Individuals with normal renal function, ingesting a regular diet, can excrete between 500 and 1000 mL/h of water.36 With the additional nonrenal losses of water as a result of sweat and insensible fluid losses, athletes should be able to consume as much as 1000 to 1500 mL/h before developing water retention and dilutional hyponatremia. Thus, although fluid ingestion is necessary to develop EAH, it is likely not sufficient except in those circumstances in which water intake is very excessive ( > 1500 mL/h).
INAPPROPRIATE AVP SECRETION
Failure to suppress AVP can markedly reduce the ability of the kidneys to excrete a water load. Under normal circumstances, ingestion of excessive water should suppress AVP, leading to production of dilute, high-volume urine (urine osmolality as low as 50 mOsm/kg and a volume of 500 to 1000 mL/h). If AVP is not suppressed appropriately with water loading, then the ability to produce dilute urine is markedly impaired (for instance, a low-level persistence of AVP can result in a fixed urine osmolality of 150 mOsm/kg and a decrease in the rate of water excretion by two-thirds as compared with a urine osmolality of 50 mOsm/kg). In fact, the available data support the concept that many athletes who experience EAH have submaximal suppression of AVP and an inappropriately high urine sodium and osmolality.23,35,37 This is similar to SIADH. There are a number of nonosmotic stimuli that lead to secretion of AVP that may be operable in endurance athletes: intense exercise itself, nausea or vomiting, hypoglycemia, and nonspecific stresses such as pain and emotion.38-40 Not all AVP release in athletes may be inappropriate, as excessive sweat losses may induce volume depletion and appropriate secretion of AVP. This appropriate AVP secretion may be important in those athletes who experience EAH along with net weight loss (Figure 1).
OTHER FACTORS
Although the combination of excessive water intake and inappropriate AVP secretion will clearly lead to hyponatremia, other factors may be operable in endurance athletes. In a study of endurance athletes running for a mean of 6 hours with ad libitum fluid intake, it was noted that even with a mean 3.8-kg mass loss, serum sodium was maintained at normal levels. Despite the loss in plasma volume in these subjects, there were elevations in the levels of brain natriuretic peptide (NT-BNP).35,40 The elevation in NT-BNP may lead to excessive losses of urine sodium and raise the risk of hyponatremia. Indeed, elevated NT-BNP concentrations and increased urinary sodium concentrations have been observed in EAH.41 A possible mechanism for maintenance of a normal serum sodium level despite weight gain is the release of sodium from internal stores.42 Up to 25% of body sodium is bound in bone (to negatively charged proteoglycan matrix) and, although not osmotically active, ispotentially recruitable into an osmotically active form.43,44 Thus, this pool could minimize the fall in serum sodium induced by overhydration or exacerbate hyponatremia if not mobilized. Whether or not impairment of this system might play a role in the development of EAH in some individuals is not clear.19 The absorption of water retained in the gastrointestinal tract at the end of a race has been suggested as a cause for an acute drop in serum sodium concentration.22,25 This may account for a transient lucid period after finishing a race followed by the acute development of clinical signs of EAHE within about 30 minutes after a competition. The breakdown of glycogen into smaller, more osmotically active molecules, such as lactate, during exercise initially increases cellular osmolality and shifts water into cells, leading to a rise in serum sodium. This may then reverse within 5 minutes after the cessation of exercise and transiently lower the serum sodium.45,46 Changes in potassium balances that serve as effective osmoles may also affect the serum sodium such that hypokalemia will lead to or exacerbate hyponatremia.
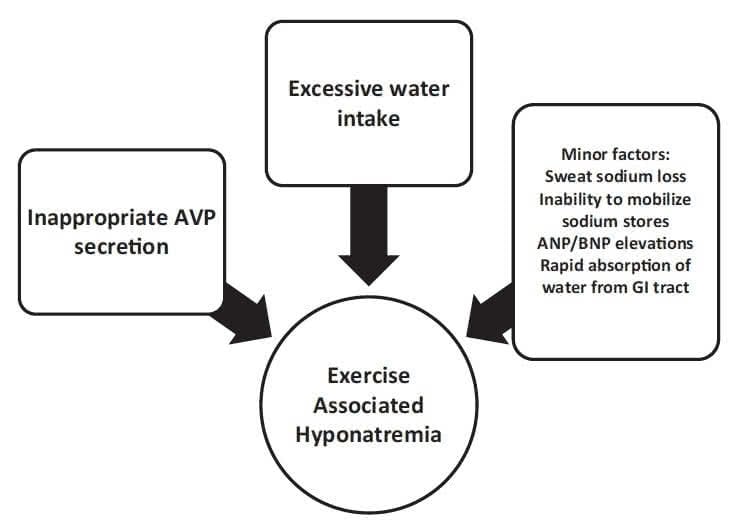
Figure 1.Exercise-associated hyponatremia pathogenesis.
ANP, atrial natriuretic peptide; AVP, arginine vasopressin; BNP, brain natriuretic peptide; GI, gastrointestinal.
The issue of whether sweat sodium loss contributes to the development of EAH remains controversial. There is a highly variable degree of sodium loss from sweat (ranging from15to65mmol/L),and compared with the general population, endurance athletes generally have lower sweat sodium levels.47,48 The direct effect of losing hypotonic sweat would be to raise the serum sodium. However, sweat loss could contribute to the development of hyponatremia if the degree of fluid loss were sufficient to produce significant volume depletion and provide a stimulus to AVP release, thereby impairing excretion of water. In this case, there would also have to be ingestion of hypotonic fluids. This scenario may explain the finding of EAH developing in some athletes with net weight loss.17-19
Risk Factors
As stated above, the major risk factor for developing EAH is excessive water intake beyond the capacity for renal water excretion.49,50 Other independent risk factors include longer race times (continuous endurance exercise lasting 4 4 hours)7,51 and a low23,25,51 or high49 body mass index (BMI). Practically speaking, smaller athletes (low BMI) who are following fluid intake guidelines designed for larger individuals and slower, unfit athletes (high BMI) drinking generous amounts of fluids while exercising at a lower intensity are at increased risk for experiencing hyponatremia during exercise. Although the incidence of women experiencing symptomatic hyponatremia appears to be greater than that of men in some environments,22,24,26,51 when adjusted for BMI and racing time, the apparent sex difference has not been shown to be statistically significant.51 Along with other nonosmotic stimuli to AVP secretion,40,52-57 nonsteroidal anti-inflammatory drugs (NSAIDs) have been implicated as a risk factor in the development of EAH22,25,58 by potentiating the water retention effects of AVP at the kidney.59,60 However, data are still conflicting,24,49 and further investigation is necessary to determine whether NSAID usage — with respect to both classification and dosages — is a clear risk factor for the development of EAH. Other medications associated with SIADH, such as selective serotonin reuptake inhibitors, may also increase the risk for EAH, but data are not conclusive.
Prevention
AVOID OVERHYDRATION
The primary strategy to prevent EAH is to avoid over- drinking during exercise. Because fluid losses through sweat and urine are highly dynamic and variable across individuals participating in a variety of outdoor activities, recommending fixed ranges of fluid intake are not appropriate. Using the sensation of thirst as a real-time guide to fluid ingestion during exercise appears safe and effective and eliminates both of the detrimental extremes of fluid balance (dehydration and overhydration).(7,61-64) Therefore, participant education on this approach to hydration during exercise is an important prevention strategy. Another strategy that has been shown to reduce the incidence of hyponatremia during endurance events is to reduce the availability of fluids along the routes of exercise.65
Recommendation: Participants should focus on avoid- ing overdrinking during exercise by drinking according to thirst, and race organizers might consider reducing the excess availability of fluids (> 3 km apart) along routes of exercise. Recommendation grade: 1B.
AVOID EXCESSIVE SODIUM SUPPLEMENTATION
Sodium supplementation during exercise has not been shown to prevent the development of hyponatremia during physical activity lasting less than 18 hours.66-69 In athletes who drink beyond thirst or fully replace 100% of body weight losses during exercise, supplemental sodium may attenuate the decline in blood sodium concentration69,70 but will not prevent the development of hyponatremia if overdrinking were to continue.67 Supplemental sodium has no effect on blood sodium concentration when athletes drink according to thirst.66-68 However, exercisers who drink insufficient amounts of fluid during exercise will often finish races with elevated blood sodium concentrations.71-74 Collectively, these results demonstrate that it is the amount of fluid ingested rather than the amount of sodium ingested during exercise that has a more pronounced effect on blood sodium concentrations as mathematically predicted else- where.75 Adverse effects associated with abnormal water retention from excessive sodium intake have been reported.76,77
Recommendation: Excessive sodium supplementation is not recommended during physical activity lasting less than 18 hours. Recommendation grade: 2B.
MONITORING BODY WEIGHT
Because over consumption of hypotonic fluids beyond the capacity to excrete any fluid excess is often key in the pathophysiology of EAH, the monitoring of body weight change is one strategy commonly used in 161-km ultramarathons to help prevent overhydration. As a result of the combination of substrate losses and the liberation of glycogen-bound water during exertion, some weight loss is appropriate during exercise. Furthermore, EAH has been reported with substantial weight loss in some environments,17-19 so weight loss is not a reliable approach for excluding the diagnosis of EAH. On the other hand, it appears as though those with EAH who have not lost weight during exercise are the most likely to become symptomatic.42,76 Therefore, in the presence of weight gain during exercise,fluid intake should be reduced, and if sodium supplementation has been taking place, this should also be curtailed until body weight returns to an appropriate level. If feasible, weight scales can be made available at organized athletic events for this purpose, but care should be taken to assure proper scale calibration and placement on solid level surfaces, and participants should be educated in proper use of body weight information.
Recommendation: Body weight can be monitored in organized events, and in the presence of weight gain during exercise, fluid and sodium intake should be reduced until weight returns to 2% to 4% of body weight loss from baseline level. Recommendation grade: 1B.
EDUCATE EVENT SUPPORT AND MEDICAL PERSONNEL
Event support staff should have a basic understanding of EAH to avoid the provision of improper hydration advice to participants because it has been previously shown that runners have a poor understanding of the relationship between drinking habits and hyponatremia.78,79 On-site medical personnel should be aware of proper treatment of EAH. This should include the recognition that hypotonic fluid replacement (intravenous or oral) should be avoided when the diagnosis of EAH is under consideration to prevent further declines in blood sodium concentration. Such education can be provided by event medical directors via prerace briefings and the use of suggested reading material or educational videotapes.
Recommendation: Event support staff should be knowledgeable so they can provide proper hydration advice, and on-site medical and emergency medical service (EMS) personnel should be educated about proper recognition and treatment of EAH. Recommendation grade: 1B.
Field Treatment
Appropriate management of EAH depends first on correctly diagnosing the condition. EAH must be routinely considered in the differential diagnosis of an individual presenting for medical attention during or shortly after exercise or strenuous activity. EAH can easily be mistaken for dehydration, heat illness, or acute altitude illnesses80,81 because of overlapping signs and symptoms if the diagnosis is not considered (Table 1).
Differentiation between dehydration and EAH is critical as provision of isotonic or hypotonic fluids is appropriate for the dehydrated athlete,82 whereas such treatment could be detrimental for an athlete with EAH, in whom the administration of these hypotonic or isotonic fluids may worsen symptoms or delay recovery.14,22,23,25,35,76,83,90 A conclusion of the Second International Exercise- Associated Hyponatremia Consensus Development Conference was that“medical directors should ensure the availability of onsite serum sodium concentration analysis.”7 When EAH is routinely considered in the differential diagnosis of a collapsed athlete and point-of-care serum sodium concentration analysis is available, the field diagnosis of EAH becomes straightforward. The reality is that on-site analysis of serum sodium concentration is not widely available at organized endurance competitions, nor is it currently feasible to widely implement. Even relatively large and established events often have no capacity for on-site blood analysis. This is also the case with most wilderness activities.
Therefore, we provide strategies for the following 2 scenarios (Figure 2).
Scenario 1: An EAH diagnosis has been made by point-of-care sodium analysis.
Scenario 2: Point-of-care sodium analysis is not available, and the diagnosis of EAH is presumed.
THERAPEUTIC OPTIONS FOR BOTH SCENARIOS
Fluids
An important element in the treatment of EAH is to avoid exacerbating the condition with improper fluid management. If EAH is clinically suspected, an assessment of volume status should be completed before treatment with intravenous (IV) fluids. It must be made clear that inappropriate IV fluid administration risks exacerbating hyponatremia with potentially devastating consequences.14,22,23,25,35,76,83–90 Thus, clear indications (such as hypotension or unstable blood pressure) should be present to support administration of IV fluids. The use of hypotonic IV fluids should be avoided. If the patient does not have clear indications for IV fluids and EAH is suspected, then fluid restriction while the patient is being transported to a medical center should be instituted.
Recommendation: Hypotonic or isotonic fluid intake should be restricted in known or suspected EAH until urination begins. Recommendation grade: 1A.
Supplemental oxygen
Hypoxemia from pulmonary edema has been reported in EAH.22 As a supportive intervention, supplemental oxygen (flow rate 2–4 L/min nasal) should be provided to treat any respiratory distress if available.
Recommendation: Respiratory symptoms should be supported with supplemental oxygen if available. Recommendation grade: 1B.
Appropriate transfer of care
The intent of field management is to stabilize the patient until they can be transferred to a definitive care medical facility. Unfortunately, EAH recognition is challenging,91 and appropriate management is not universally understood. Therefore, when transferring care, it is critical to relay the potential diagnosis of EAH and to caution the transport team about the dangers of aggressive IV hydration with isotonic or hypotonic fluids. Ideally, an IV saline lock should be placed for EMS transport, and the provision of IV fluids should be based on clear indications of marked hypovolemia such as sustained hypotension. However, if the transport team insists on provision of isotonic or hypotonic fluids, they should be cautioned that a patient with EAH could experience worsening symptoms with this intervention. If symptoms worsen in this scenario, IV fluids should be stopped, and consideration of immediate hypertonic (3%) saline administration should occur.
Recommendation: When transferring care, receiving caregivers should be alerted to the potential diagnosis of EAH and appropriate fluid management. Recommendation grade: 1B.
SPECIFIC RECOMMENDATIONS—SCENARIO 1 (BLOOD SODIUM ESTIMATION IS AVAILABLE)
Clinical assessment
The portability of point-of-care testing devices means that they may be available to confirm a diagnosis of EAH, for instance at a mass-participation wilderness sporting event or on a well-equipped expedition.30,92–94 In general, a sodium level of 130 mmol/L or higher will be minimally symptomatic or asymptomatic, whereas levels below this are increasingly likely to be symptomatic.14,22,23,25,26,35,76,83,85–90,92,93 Symptoms and signs of EAHE (ranging from headache and nausea or vomit- ing to confusion and lethargy) are key elements in making the diagnosis. Although the early symptoms of EAH may be nonspecific, the presence of altered mental status, coma, seizures, or respiratory distress (suggesting pulmonary edema)supports the diagnosis of EAHE7,22,25,83 and should be promptly recognized.
Recommendation: A rapid assessment for signs and symptoms of cerebral edema or noncardiogenic pulmonary edema should be made in all patients with possible EAH. Recommendation grade: 1B.
Table 1. Signs and symptoms of exercise-associated hyponatremia and heat illness or altitude illness
General
| Sign/Symptom | EAH | Heat illness | AMS, HACE, or HAPE |
|---|---|---|---|
| Fatigue/weakness | Possible | Possible | Likely |
| Increased thirst | Possible | Likely | Possible |
| Temperature: Elevated | Possible | Present | Not present |
| Tachycardia | Possible | Likely | Possible |
| Orthostasis | Possible | Likely | Possible |
| Nausea/vomiting | Possible | Possible | Possible |
| Headache/dizziness | Possible | Possible | Present |
| Blurred vision | Possible | Possible | Possible |
| Confusion/disorientation | Possible | Possible | Possible |
| Obtundation | Possible | Possible | Possible |
| Seizure | Possible | Possible | Possible |
| Coma | Possible | Possible | Possible |
| Respiratory distress | Possible | Not present | Possible |
| Oliguria | Possible | Likely | Possible |
| Diuresis | Possible | Not present | Possible |
AMS, acute mountain sickness; EAH, exercise-associated hyponatremia; HACE, high altitude cerebral edema; HAPE, high altitude pulmonary edema.
Developing signs of cerebral or pulmonary edema (non-cardiogenic) signify an urgent medical condition requiring emergent care. In such situations, urgent blood sodium measurement is invaluable in guiding initial therapy.94
Recommendation: When point-of-care sodium analysis is available in the field and EAH is suspected, blood sodium measurement should be obtained as rapidly as possible. Recommendation grade: 1B.
Hypertonic saline
It is possible to commence and indeed even complete treatment for EAH in the field.92-94 Individuals with EAH who are neurologically stable can be advised to limit fluid intake and consume salty snacks, soups or bouillon, or a small volume of hypertonic fluid until the onset of urination. They should be observed for at least 60 minutes during the initial post exercise period because water remaining in the gastrointestinal tract can be quickly absorbed at the cessation of exercise and result in rapid development of symptoms from EAH.22,25,83 More urgent medical attention, including planning for transfer to definitive care, is required if signs or symptoms of EAH develop. Once any neurological symptoms more serious than headache develop, regardless of the degree of hyponatremia, treatment with hypertonic saline is indicated. When able to tolerate oral intake, a hypertonic (approximately 9% saline) solution of concentrated broth (3–4 bouillon cubes in 125 mL [1/2 cup] of water) would be an appropriate initial treatment.92–94
Recommendation: Oral hypertonic saline solutions are an appropriate intervention in the field for cases of EAH when oral intake is possible. Recommendation grade: 1B.
If the individual is unable to tolerate oral intake, or when there is no improvement or symptoms worsen with oral hypertonic saline, the recommended treatment is a 100-mL bolus of 3% hypertonic saline infused through a peripheral vein in less than 60 seconds. This can be repeated 2 additional times at 10-minute intervals if there is no clinical improvement.7 Experience has proven this treatment to be without untoward symptoms at the infusion site (no burning, phlebitis, or residual discomfort) and no risk of osmotic demyelination or central pontine myelinolysis.7,22,23,25,35,76,83,85–87,92,93
Recommendation: Symptomatic biochemically confirmed EAH can be treated in the field with a 100-mL bolus of 3% hypertonic saline, which can be repeated twice at 10-minute intervals (3 doses in total) with the aim of acutely increasing serum sodium concentration by about 4 to 5 mmol/L and reversing cerebral edema in the setting of acute hyponatremia. Recommendation grade: 1B.
SPECIFIC RECOMMENDATIONS—SCENARIO 2 (BLOOD SODIUM ESTIMATION IS NOT AVAILABLE)
When the capacity for on-site serum sodium measurement is not available, the decision-making process becomes challenging. Unfortunately, the possible signs and symptoms that can be present with EAH are quite similar to those present with heat illness, dehydration, or acute mountain sickness (Table 1). Furthermore, there were no differences between those experiencing mild EAH and those not experiencing EAH after a 161-km ultramarathon in terms of various individual characteristics, signs, and symptoms.91 Even oliguria, which would be typical of the dehydrated state, is also commonly seen with EAH when AVP secretion is part of the pathophysiological mechanism leading to a highly concentrated, low-volume urine output.23
In some environments in which overhydration is a key feature in the underlying etiology of EAH, those experiencing EAH have been shown to be more likely to lose less weight or to gain weight during the exercise when compared with those not experiencing EAH.42 However, in other environments, it is not at all uncommon for those with EAH to have considerable weight loss, suggesting other mechanisms in the development of EAH.17-19 Therefore, changes in body weight are not universally helpful in making the diagnosis of EAH. This unfortunately means that the only reliable method of diagnosing EAH at present is through measurement of serum sodium concentration. On the other hand, it now appears that those developing symptomatic EAH generally have inadequate weight loss during the exercise.42,76 So, if it is known that a symptomatic athlete has gained weight or lost little weight during the exercise, clinical suspicion of EAH may be raised.
Figure 2.Algorithm for exercise-associated hyponatremia (EAH) field management.
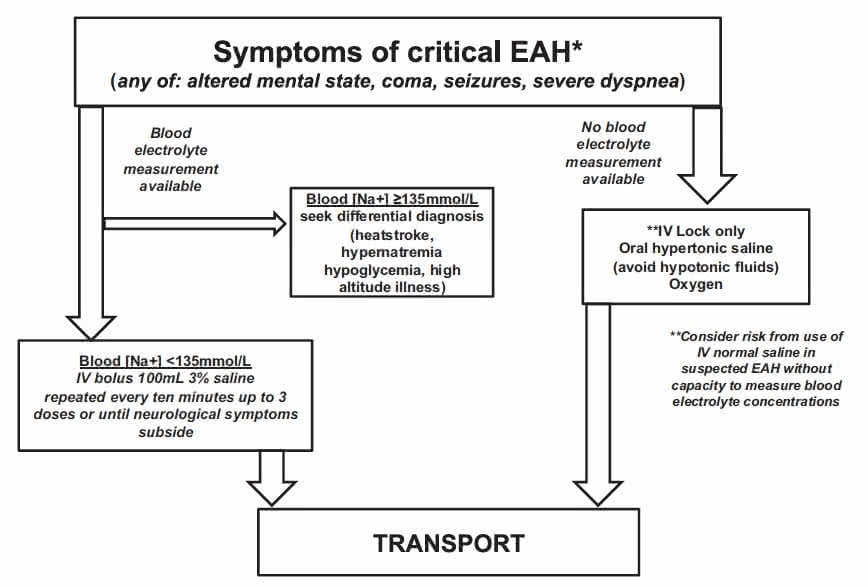
Asymptomatic EAH is generally not seen unless blood tests are obtained for other reasons. When only mild symptoms are present, treatment can be with either fluid restriction or oral hypertonic solutions (if tolerated) until the onset of urination.
Fluid restriction
A high clinical suspicion of symptomatic EAH necessitates fluid restriction and salt supplementation. Certainly in events such as long ultramarathon races in which the incidence of EAH may be high (30%–51%),13,17-19,92 one should resist treating athletes with IV hypotonic or isotonic saline without certainty that they do not have EAH.14,22,23,25,35,76,83,85-90 However, fluid restriction is contraindicated in the case of dehydration and rhabdomyolysis with impending acute kidney injury.82 Therefore, in the situation in which the diagnosis of EAH is uncertain, the potential benefits of fluid restriction if the individual has EAH must be weighed against the potential harm that could result when the individual might have dehydration, rhabdomyolysis, and impending acute renal failure.
Recommendation: Hypotonic or isotonic fluids should be restricted in suspected EAH with consideration of the potential harm that could result from fluid restriction if the diagnosis is incorrect. Recommendation grade: 1C.
Hypertonic saline
In the event of neurological deterioration without access to rapid determination of serum sodium concentration, the use of IV hypertonic saline, if available, should be considered for presumed EAH. Such an intervention carries minimal potential risks, and the benefit that could be derived from a bolus of hypertonic saline of approximately 51 mmol of sodium for fluid volume expansion and the limited effect it would have on increasing blood sodium concentration suggest that the risk is low, even under conditions of dehydration and hypernatremia. When the patient is neurologically stable, oral sodium with limited fluid has been demonstrated to be an appropriate treatment.92-94 In the field, this could be prepared by dissolving 3 to 4 bouillon cubes in 125 mL (1/2 cup) of water (approximately 9% saline).
Recommendation: IV hypertonic saline (100-mL bolus of 3% hypertonic saline, which can be repeated twice at 10-minute intervals) is an appropriate consideration in suspected EAH with neurological deterioration, whereas an oral hypertonic saline solution would be an appropriate consideration in suspected mild EAH. Recommendation grade: 1C.
Emergency transport
When point-of-care serum sodium concentration cannot be determined and any attempted field treatment has been insufficiently successful, emergency transport to a definitive care facility should be expedited. Organized endurance exercise events that do not have the on-site capacity for measurement of serum sodium concentration and treatment with hypertonic saline should have pre- arranged emergency transport systems. Local emergency department physicians and transport personnel should also be educated about EAH in advance of the event.
Recommendation: The assurance that an emergency transport system is in place is critical when point-of-care serum sodium measurement will not be available or treatment with hypertonic saline will not be feasible. Recommendation grade: 1C.
IMMEDIATE MEDICAL CARE IN-HOSPITAL ASSESSMENT
The medical care of suspected EAH in hospital is presumed to occur in a facility that has the capacity to measure an urgent sodium level either by point-of-care testing or in a hospital laboratory. The sodium level and a clinical assessment for signs of cerebral edema are the key factors that will determine urgent treatment. The primary intervention (when indicated) is the use of IV hypertonic saline, usually as a 3% solution, to acutely increase serum sodium and reduce cerebral edema, whereas the role of other therapeutic agents such as vasopressin receptor antagonists (vaptans), urea, oral saline solutions, and diuretics in the treatment of acute symptomatic cases of EAH has not been firmly established.8 See Table 2 for a summary of critical steps for immediate medical care in a receiving hospital.
Urgent sodium estimation
The in-hospital diagnosis of EAH is made in an appropriate clinical context, whether or not signs or symptoms are present, by the determination of a sodium level below the normal reference range. In general, a sodium level of 130 mmol/L or higher will be minimally symptomatic or asymptomatic,7,8,92,93 whereas levels below this are likely to be symptomatic.14,22,23,25,35,76,83,85–90 Developing signs of cerebral edema signify an urgent medical condition requiring emergent care.7,22,23,25,76,83,94
Recommendation: With suspected EAH, and particularly in those with altered mental status, sodium estimation should be obtained as rapidly as possible after hospital arrival. Recommendation grade: 1B.
Assessment for cerebral and pulmonary edema
Symptoms and signs of cerebral edema are a key element in making the diagnosis of severe or clinically significant EAH. Although the early symptoms of EAH may be nonspecific, the presence of altered mental state, coma, seizures, or respiratory distress (suggesting pulmonary edema) indicates severe EAH.7,22,23,25,76,83 Such an assessment is made clinically at the bedside. It does not require imaging or scans and should never delay the use of IV hypertonic saline when indicated (see sub-sequent sections).
Recommendation: A rapid assessment for signs and symptoms of cerebral edema or noncardiogenic pulmonary edema should be made in all patients with possible EAH. Recommendation grade: 1B.
Other laboratory testing
Although not essential to guide initial therapy, there are other laboratory tests that can help to better delineate the pathophysiology of EAH in an individual patient and help guide subsequent treatment if more prolonged in-hospital care is required.4 These tests may help to differentiate euvolemic from hypovolemic EAH and the role of AVP and brain natriuretic peptide in its pathogenesis.7,8,23,41,95 Such tests are best taken before therapy is commenced even if they are only stored for subsequent analysis. However, such testing should never delay the use of IV hypertonic saline when indicated (see subsequent sections).
Recommendation: When possible, urine for sodium and osmolality and blood for osmolality should be obtained before commencement of treatment. Recommendation grade: 2C.
Table 2.Summary of acute hospital assessment and management of EAH
Assessment
- Urgent measurement of blood sodium by the most rapidly available means
- Assess for clinical signs suggestive of developing cerebral edema
- Obtain and store specimens if possible for later analysis of blood serum osmolality and urine sodium and osmolality
Management
- Supplemental oxygen to maintain oxygen saturation above 95%
- Restrict fluids (both IV and oral) until onset of urination
- Avoid IV normal saline until sodium correction is initiated
- Thereafter normal saline may be required for hypovolemic shock or in renal protection therapy for rhabdomyolysis
- In severe EAH (signs of cerebral edema or serum sodium < 125 mmol/L) administer IV 3% hypertonic saline as a 100-mL bolus repeated twice at 10-minute intervals aiming to reverse cerebral edema
- Aim to increase serum sodium by approximately 4 to 5 mmol/L or until neurological symptoms are reversed by active treatment, then allow the remaining correction to occur spontaneously via urinary free water excretion
Fluid restriction
Mild or asymptomatic EAH (essentially a biochemical- only diagnosis) will usually resolve without treatment during a period of observation. Hypotonic fluids, whether taken orally or given IV, will generally worsen the situation, especially before the onset of urination. IV normal saline will worsen EAH acutely in the presence of osmotically inappropriate (nonosmotic) AVP secretion,14,22,23,25,35,76,83,85-90 but may be required later in specific clinical contexts such as the prevention of renal injury in rhabdomyolysis or the treatment of hypovolemic shock.
Recommendation: Oral and IV hypotonic or isotonic hydration should be avoided early in the management of EAH although it may be appropriate in certain clinical contexts once sodium correction has been initiated or hypovolemia is biochemically confirmed (by elevated blood urea nitrogen and urine sodium less than 30 mmol/ L). Recommendation grade: 1B.
Hypertonic saline
The most commonly available form of IV hypertonic saline is a 3% solution.4,25,76,83,92-94 Hypertonic saline will acutely raise the serum sodium, resulting in a fluid shift that will decrease cerebral edema. A 100-mL solution of 3% hypertonic saline contains 51 mmol of sodium and in the average adult would be expected to increase the serum sodium by 1 to 2 mmol/L. It is used whenever there are signs of significant cerebral edema in EAH.7,22,76,83,94 It may also be indicated in severe biochemical hyponatremia (o125 mmol/L) when initially presenting as asymptomatic or mildly symptomatic, as these cases have been known to progress to EAHE.23,76 When using IV hypertonic saline, the aim is not to normalize the serum sodium concentration but rather to reverse cerebral edema while preventing or treating the life-threatening consequences of EAHE.4,7,94 In general, this will require an increase in the sodium level of about 4 to 5 mmol/L. Thereafter, further normalization of sodium is not urgent and may be best allowed to occur spontaneously through suppression of nonosmotic AVP secretion and resultant urinary free water excretion. The use of IV hypertonic saline in EAH appears to be safe,7,92,93 with no recorded cases of osmotic demyelination known to have occurred as opposed to the situation with rapid correction of chronic hyponatremia.7,8
Recommendation: In hospital, severe biochemically confirmed or symptomatic EAH should be treated with a 100-mL bolus of 3% hypertonic saline, which can be repeated twice at 10-minute intervals (3 doses in total), with the aim of acutely increasing serum sodium concentration by about 4 to 5 mmol/L and reversing cerebral edema. Recommendation grade: 1A.
Supplemental oxygen
Although the major manifestation of EAH is cerebral, pulmonary manifestations can occur.22 Hypoxemia, which may worsen cerebral injury, should be avoided, but hyperoxia may also have detrimental effects.96
Recommendation: Supplemental oxygen to maintain an oxygenation saturation of 95% should be provided to treat hypoxemia from pulmonary edema when evident. Recommendation grade: 1B.
Conclusions
Exercise-associated hyponatremia has a complex pathogenesis and multifactorial etiology. It can result in devastating outcomes to participants in both organized or individual endurance activities in urban or in remote backcountry environments.
Preventing EAH is the key factor in protecting participants in endurance events and other wilderness activities. Currently, there is no one recommendation that fits all individuals for fluid and salt consumption during endurance events, although prudent general guidelines include drinking to thirst and specifically avoiding excessive fluid intake. There is an ongoing need for education to ensure that participants understand the risk of overhydration. Furthermore, a knowledge gap persists internationally among practitioners and prehospital EMS personnel about the assessment and treatment of EAH, which is compounded by the nonspecific nature of many of the signs and symptoms of EAH. The typical field response is to administer rapid isotonic IV fluids to endurance activity participants in the suspicion they are dehydrated. However, such universal treatment may result in increased morbidity and mortality in the EAH patient. A Supplementary Evidence Table 2is available online.
Acknowledgments
The authors thank the Wilderness Medical Society for the assistance provided for two Exercise-Associated Hyponatremia panel presentations at the Desert Medicine conference, Tucson, Arizona, November 2011, and at the 6th World Congress on Wilderness Medicine, Whistler, British Columbia, July 2012. This material is the result of work supported with resources and the use of facilities at the Veterans Administration Northern California Health Care System. The contents reported here do not represent the views of the Department of Veterans Affairs or the United States Government.
Supplementary tables
Supplementary ACCP Table 1 and Evidence Table 2 are available online atdoi:10.1016/j.wem.2014.08.009.
References
- Noakes TD, Goodwin N, Rayner BL, Branken T, Taylor RK. Water intoxication: a possible complication during endurance exercise.Med Sci Sports Exerc. 1985;17:370– 375.
- Montain SJ, Sawka MN, Wenger CB. Hyponatremia associated with exercise: risk factors and pathogenesis. Exerc Sport Sci Rev. 2001;29:113– 117.
- Speedy DB, Noakes TD, Schneider C. Exercise-associated hyponatremia: a review. Emerg Med (Fremantle). 2001;13:17– 27.
- Rosner MH, Kirven J. Exercise-associated hyponatremia. Clin J Am Soc Nephrol. 2007;2:151– 161.
- Rosner MH. Exercise-associated hyponatremia. Semin Nephrol. 2009;29:271– 281.
- Rosner MH, Bennett B, Hew-Butler T, Hoffman MD. Exercise-associated hyponatremia. In: Simon EE, ed. Hyponatremia: Evaluation and Treatment. New York, NY: Springer; 2013:175– 192.
- Hew-Butler T, Ayus JC, Kipps C, et al. Statement of the Second International Exercise-Associated Hyponatremia Consensus Development Conference, New Zealand, 2007.Clin J Sport Med. 2008;18:111– 121.
- Hew-Butler T, Almond C, Ayus JC, et al. Exercise- Associated Hyponatremia (EAH) Consensus Panel. Consensus statement of the 1st International Exercise- Associated Hyponatremia Consensus Development Con- ference, Cape Town, South Africa 2005.Clin J Sport Med. 2005;15:208– 213.
- Backer HD, Shopes E, Collins SL. Hyponatremia in recreational hikers in Grand Canyon National Park. J Wilderness Med. 1993;4:391– 406.
- Basnyat B, Sleggs J, Spinger M. Seizures and delirium in a trekker: the consequences of excessive water drinking? Wilderness Environ Med. 2000;11:69– 70.
- Rothwell SP, Rosengren DJ. Severe exercise-associated hyponatremia on the Kokoda Trail, Papua New Guinea. Wilderness Environ Med. 2008;19:42– 44.
- Zafren K. Hyponatremia in a cold environment.Wilder- ness Environ Med. 1998;9:54– 55.
- Stuempfle KJ, Lehmann DR, Case HS, et al. Hypona- tremia in a cold weather ultraendurance race.Alaska Med. 2002;44:51– 55.
- Coler C, Hoffman MD, Towle G, Hew-Butler T. Hypo- natremia in an 85-year-old hiker: when depletion plus dilution produces delirium. Wilderness Environ Med. 2012;23:153– 157.
- Rogers IR, Hew-Butler T. Exercise-associated hyponatre- mia: overzealousfluid consumption.Wilderness Environ Med. 2009;20:139– 143.
- Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force.Chest. 2006;129:174– 181.
- Lebus DK, Casazza GA, Hoffman MD, Van Loan MD. Can changes in body mass and total body water accurately predict hyponatremia after a 161-km running race?Clin J Sport Med. 2010;20:193– 199.
- Hoffman MD, Stuempfle KJ, Rogers IR, Weschler LB, Hew-Butler T. Hyponatremia in the 2009 161-km Western States Endurance Run. Int J Sports Physiol Perform. 2012;7:6– 10.
- Hoffman MD, Hew-Butler T, Stuempfle KJ. Exercise-associated hyponatremia and hydration status in 161-km ultramarathoners.Med Sci Sports Exerc. 2013;45:784– 791.
- Noakes T.Waterlogged: The Serious Problem of Over-hydration in Endurance Sports.Champaign, IL: Human Kinetics; 2012.
- Kipps C, Sharma S, Pedoe DT. The incidence of exercise- associated hyponatraemia in the London marathon.Br J Sports Med. 2011;45:14– 19.
- Ayus JC, Varon J, Arieff AI. Hyponatremia, cerebral edema, and noncardiogenic pulmonary edema in marathon runners.Ann Intern Med. 2000;132:711– 714.
- Siegel AJ, Verbalis JG, Clement S, et al. Hyponatremia in marathon runners due to inappropriate arginine vasopres- sin secretion.Am J Med. 2007;120:461.e11–e17.
- Hew TD, Chorley JN, Cianca JC, Divine JG. The incidence, risk factors, and clinical manifestations of hyponatremia in marathon runners.Clin J Sport Med. 2003;13:41– 47.
- Davis DP, Videen JS, Marino A, et al. Exercise-associated hyponatremia in marathon runners: a two-year experience. J Emerg Med. 2001;21:47– 57.
- Speedy DB, Noakes TD, Rogers IR, et al. Hyponatremia in ultradistance triathletes. Med Sci Sports Exerc. 1999;31:809– 815.
- Lee JK, Nio AQ, Ang WH, et al. First reported cases of exercise-associated hyponatremia in Asia. Int J Sports Med. 2011;32:297– 302.
- Glace B, Murphy C. Severe hyponatremia develops in a runner following a half-marathon.JAAPA. 2008;21:27– 29.
- Shapiro SA, Ejaz AA, Osborne MD, Taylor WC. Moder- ate exercise-induced hyponatremia. Clin J Sport Med. 2006;16:72– 73.
- Backer HD, Shopes E, Collins SL, Barkan H. Exertional heat illness and hyponatremia in hikers.Am J Emerg Med. 1999;17:532– 539.
- Noe RS, Choudhary E, Cheng-Dobson J, Wolkin AF, Newman SB. Exertional heat-related illnesses at the Grand Canyon National Park, 2004–2009.Wilderness Environ Med. 2013;24:422– 428.
- Gardner JW. Death by water intoxication. Mil Med. 2002;167:432– 434.
- Garigan TP, Ristedt DE. Death from hyponatremia as a result of acute water intoxication in an Army basic trainee. Mil Med. 1999;164:234– 238.
- O’Donnell FL, ed. Army Medical Surveillance Activity. Update: exertional hyponatremia, active component, U.S. Armed Forces, 1999–2012.Medical Surveillance Monthly Report. 2013:20;23–28.
- Zelingher J, Putterman C, Ilan Y, et al. Case series: hyponatremia associated with moderate exercise.Am J Med Sci. 1996;311:86– 91.
- Rose BD, Post TW.Clinical Physiology of Acid-Base and Electrolyte Disorders. 5th ed. New York, NY: McGraw Hill; 2001.
- Hew-Butler T, Dugas JP, Noakes TD, Verbalis JG. Changes in plasma arginine vasopressin concentrations in cyclists participating in a 109-km cycle race.Br J Sports Med. 2010;44:594– 597.
- Rowe JW, Shelton RL, Helderman JH, Vestal RE, Robertson GL. Influence of the emetic reflex on vaso- pressin release in man.Kidney Int. 1979;16:729– 735.
- Baylis PH, Zerbe RL, Robertson GL. Arginine vasopressin response to insulin-induced hypoglycemia in man.J Clin Endocrinol Metab. 1981;53:935– 940.
- Hew-Butler T, Jordaan E, Stuempfle KJ, et al. Osmotic and nonosmotic regulation of arginine vasopressin during prolonged endurance exercise.J Clin Endocrinol Metab. 2008;93:2072– 2078.
- Harris G, Reid S, Sikaris K, McCrory P. Hyponatremia is associated with higher NT-proBNP than normonatremia after prolonged exercise.Clin J Sport Med. 2012;22:488– 494.
- Noakes TD, Sharwood K, Speedy D, et al. Three independent biological mechanisms cause exercise- associated hyponatremia: evidence from 2,135 weighed competitive athletic performances.Proc Natl Acad Sci USA. 2005;102:18550– 18555.
- Edelman IS, James AH, Brooks L, Moore FD. Body sodium and potassium. IV. The normal total exchangeable sodium; its measurement and magnitude.Metabolism. 1954;3:530– 538.
- Edelman IS, James AH, Baden H, Moore FD. Electrolyte composition of bone and the penetration of radiosodium and deuterium oxide into dog and human bone.J Clin Invest. 1954;33:122– 131.
- Halperin ML, Kamel KS, Sterns R. Hyponatremia in marathon runners.N Engl J Med. 2005;353:427– 428.
- Lindinger MI, Heigenhauser GJ, McKelvie RS, Jones NL. Blood ion regulation during repeated maximal exercise and recovery in humans. Am J Physiol. 1992;262(1 Pt 2): R126–R136.
- Buono MJ, Ball KD, Kolkhorst FW. Sodium ion concen- tration vs. sweat rate relationship in humans. J Appl Physiol. 2007;103:990– 994.
- Buono MJ, Sjoholm NT. Effect of physical training on peripheral sweat production.J Appl Physiol. 1988;65:811– 814.
- Speedy DB, Noakes TD, Boswell T, Thompson JM, Rehrer N, Boswell DR. Response to afluid load in athletes with a history of exercise induced hyponatremia.Med Sci Sports Exerc. 2001;33:1434– 1442.
- Noakes TD, Wilson G, Gray DA, Lambert MI, Dennis SC. Peak rates of diuresis in healthy humans during oralfluid overload.S Afr Med J. 2001;91:852– 857.
- Almond CS, Shin AY, Fortescue EB, et al. Hyponatremia among runners in the Boston Marathon.N Engl J Med. 2005;352:1550– 1556.
- Hew-Butler T, Hoffman MD, Stuempfle KJ, Rogers IR, Morgenthaler NG, Verbalis JG. Changes in copeptin and bioactive vasopressin in runners with and without hypo- natremia.Clin J Sport Med. 2011;21:211– 217.
- Schmidt W, Rojas J, Böning D, Bernal H, Garcia S, Garcia O. Plasma-electrolytes in natives tohypoxia after marathon races at different altitudes.Med Sci Sports Exerc. 1999;31:1406– 1413.
- Hellmann K, Weiner JS. Antidiuretic substance in urine following exposure to high temperatures.J Appl Physiol. 1953;6:194– 198.
- Grant SM, Green HJ, Phillips SM, Enns DL, Sutton JR. Fluid and electrolyte hormonal responses to exercise and acute plasma volume expansion.J Appl Physiol. 1996;81: 2386 – 2392.
- Stebbins CL, Symons JD, McKirnan MD, Hwang FF. Factors associated with vasopressin release in exercising swine.Am J Physiol. 1994;266(1 Pt 2):R118–R124.
- Siegel AJ. Exercise-associated hyponatremia: role of cytokines.Am J Med. 2006;119(7 Suppl 1):S74–S78.
- Wharam PC, Speedy DB, Noakes TD, Thompson JM, Reid SA, Holtzhausen LM. NSAID use increases the risk of developing hyponatremia during an Ironman triathlon. Med Sci Sports Exerc. 2006;38:618– 622.
- Baker J, Cotter JD, Gerrard DF, Bell ML, Walker RJ. Effects of indomethacin and celecoxib on renal function in athletes.Med Sci Sports Exerc. 2005;37:712– 717.
- Walker RJ, Fawcett JP, Flannery EM, Gerrard DF. Indome- thacin potentiates exercise-induced reduction in renal hemody- namics in athletes.Med Sci Sports Exerc. 1994;26:1302– 1306.
- Hew-Butler T, Verbalis JG, Noakes TD, International Marathon Medical Directors Association. Updated fluid recommendation: position statement from the International Marathon Medical Directors Association (IMMDA).Clin J Sport Med. 2006;16:283– 292.
- Cheuvront SN, Haymes EM. Ad libitumfluid intakes and thermoregulatory responses of female distance runners in three environments.J Sports Sci. 2001;19:845– 854.
- Armstrong LE, Maresh CM, Gabaree CV, et al. Thermal and circulatory responses during exercise: effects of hypohydration, dehydration, and water intake. J Appl Physiol. 1997;82:2028– 2035.
- Hoffman MD, Stuempfle KJ. Hydration strategies, weight change and performance in a 161-km ultramarathons.Res Sports Med. 2014;22:213– 225.
- Speedy DB, Rogers IR, Noakes TD, et al. Diagnosis and prevention of hyponatremia at an ultradistance triathlon. Clin J Sport Med. 2000;10:52– 58.
- Speedy DB, Thompson JM, Rodgers I, Collins M, Shar- wood K, Noakes TD. Oral salt supplementation during ultradistance exercise.Clin J Sport Med. 2002;12:279– 284.
- Hew-Butler TD, Sharwood K, Collins M, Speedy D, Noakes T. Sodium supplementation is not required to maintain serum sodium concentrations during an Ironman triathlon.Br J Sports Med. 2006;40:255– 259.
- Twerenbold R, Knechtle B, Kakebeeke TH, et al. Effects of different sodium concentrations in replacementfluids during prolonged exercise in women.Br J Sports Med. 2003;37:300– 303.
- Barr SI, Costill DL, Fink WJ. Fluid replacement during prolonged exercise: effects of water, saline, or nofluid. Med Sci Sports Exerc. 1991;23:811– 817.
- Vrijens DM, Rehrer NJ. Sodium-free fluid ingestion decreases plasma sodium during exercise in the heat.J Appl Physiol. 1999;86:1847– 1851.
- Röcker L, Kirsch KA, Heyduck B, Altenkirch HU. Influence of prolonged physical exercise on plasma volume, plasma proteins, electrolytes, and fluid- regulating hormones.Int J Sports Med. 1989;10:270– 274.
- Beckner GL, Winsor T. Cardiovascular adaptations to prolonged physical effort.Circulation. 1954;9:835– 846.
- Riley WJ, Pyke FS, Roberts AD, England JF. The effect of long-distance running on some biochemical variables.Clin Chim Acta. 1975;65:83– 89.
- Åstrand PO, Saltin B. Plasma and cell volume after prolonged severe exercise.J Appl Physiol. 1964;19:829– 832.
- Weschler LB. Exercise-associated hyponatraemia: a math- ematical review.Sports Med. 2005;35:899– 922.
- Hoffman MD, Stuempfle KJ, Sullivan K, Weiss RH. Exercise-associated hyponatremia with exertional rhabdo- myolysis: importance of proper treatment.Clin Nephrol. 2014 Jun 16. [Epub ahead of print].
- Luks AM, Robertson HT, Swenson ER. An ultracyclist with pulmonary edema during the Bicycle Race Across America.Med Sci Sports Exerc. 2007;39:8– 12.
- Williams J, Tzortziou Brown V, Malliaras P, Perry M, Kipps C. Hydration strategies of runners in the London Marathon.Clin J Sport Med. 2012;22:152– 156.
- Winger JM, Dugas JP, Dugas LR. Beliefs about hydration and physiology drive drinking behaviours in runners.Br J Sports Med. 2011;45:646– 649.
- Ayus JC, Moritz ML. Exercise-associated hyponatremia masquerading as acute mountain sickness: are we missing the diagnosis?Clin J Sport Med. 2008;18:383– 386.
- Sucholeiki R. Heatstroke.Semin Neurol. 2005;25:307– 314.
- Patel DR, GyamfiR, Torres A. Exertional rhabdomyolysis and acute kidney injury.Phys Sportsmed. 2009;37:71– 79.
- Frizzell RT, Lang GH, Lowance DC, Lathan SR. Hypona- tremia and ultramarathon running.JAMA. 1986;255:772– 774.
- Bennett BL, Hew-Butler T, Hoffman MD, Rogers IR, Rosner MH. In reply to Clinical practice guidelines for treatment of exercise-associated hyponatremia.Wilderness Environ Med. 2013;24:468– 471.
- Hew-Butler TD, Boulter J, Bhorat R, Noakes TD. Avoid adding insult to injury—correct management of sick female endurance athletes.S Afr Med J. 2012;102:927– 930.
- Kormann R, Philippart F, Hubert S, Bruel C. Marathon runner with acute hyponatremia: a neurological disorder. Case Rep Emerg Med. 2012;2012:342760. doi: 10.1155/ 2012/342760. Epub 2012 May 31.
- Reynolds CJ, Cleaver BJ, Finlay SE. Exercise associated hyponatraemia leading to tonic-clonic seizure.BMJ Case Rep. 2012 Aug 27;2012.
- Severac M, Leplatois T, Ichai C. A near-fatal case of exercise-associated hyponatremia. Am J Emerg Med. 2014;32:813.e1–e2.
- Spano SJ, Reagle Z, Evans T. Symptomatic hypotonic hyponatremia presenting at high altitude. Wilderness Environ Med. 2014;25:69– 74.
- Thompson J-A, Wolff AJ. Hyponatremic encephalopathy in a marathon runner.Chest. 2003;124:313S.
- Hoffman MD, Fogard K, Winger J, Hew-Butler T, Stuempfle KJ. Characteristics of 161-km ultramarathon finishers developing exercise-associated hyponatremia.Res Sports Med. 2013;21:164– 175.
- Rogers IR, Hook G, Stuempfle KJ, Hoffman MD, Hew-Butler T. An intervention study of oral versus intra- venous hypertonic saline administration in ultramarathon runners with exercise-associated hyponatremia: a prelimi- nary randomized trial. Clin J Sport Med. 2011;21: 200 – 203.
- Owen BE, Rogers IR, Hoffman MD, et al. Efficacy of oral versus intravenous hypertonic saline in runners with hyponatremia.J Sci Med Sport. 2014;17:457– 462.
- Siegel AJ, d’Hemecourt P, Adner MM, Shirey T, Brown JL, Lewandrowski KB. Exertional dysnatremia in col- lapsed marathon runners: a critical role for point-of-care testing to guide appropriate therapy.Am J Clin Pathol. 2009;132:336– 340.
- Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations.Am J Med. 2007;120( Suppl 1):S1–S21.
- Iscoe S, Beasley R, Fisher JA. Supplementary oxygen for nonhypoxemic patients: O2 much of a good thing?Crit Care. 2011;15:305.
