
Wilderness Medical Society Clinical Practice Guidelines for Diabetes Management
Karin D.Van Baak MD, Laura M.Nally MD, Ryan T. Finigan MD, Carrie L. Jurkiewicz MD, Andre M. Burnier MD, Barry P.Conrad MPH, RD, Morteza Khodaee MD, MPH, Grant S. Lipman MD
The Wilderness Medical Society convened an expert panel in 2018 to develop a set of evidence-based guidelines for the treatment of type 1 and 2 diabetes, as well as the recognition, prevention, and treatment of complications of diabetes in wilderness athletes. We present a review of the classifications, pathophysiology, and evidence-based guidelines for planning and preventive measures, as well as best practice recommendations for both routine and urgent therapeutic management of diabetes and glycemic complications. These recommendations are graded based on the quality of supporting evidence and balance between the benefits and risks or burdens for each recommendation.
Introduction
In 2015, the Centers for Disease Control & Prevention estimated that 30 million individuals in the United States (9% of the population) have diabetes.1 Athletes with diabetes are undertaking ever-expanding wilderness challenges. People self-identified as having diabetes represented 7% of 3000 surveyed ultramarathon runners,2 and 19 individuals with type 1 diabetes have entered or completed the high altitude Leadville 161 km (100 mi) ultramarathon trail and mountain bike race.3 To date, at least 3 individuals with diabetes have successfully summited Mount Everest.4
Hypoglycemia and hyperglycemia can be catastrophic in resource-limited environments, and glycemic control is more challenging in extreme conditions, requiring additional monitoring, treatment adjustments, and careful pre-trip planning.4 Athletes with diabetes have been shown to have a lower maximum oxygen consumption ,5 impaired heat loss,6,7 higher risk of acute kidney injury,8 and higher risk of infections,9 among other complications.9 Athletes with diabetes may also be at increased risk of altitude-related illness.10 However, the benefits of exercise for people with diabetes are numerous and well documented, including improved HbA1c,11,12 lower body mass index,12 improved blood pressure levels,13 improved lipid profiles,13 and decreased all-cause mortality.14
The Wilderness Medical Society convened an expert panel to develop a set of evidence-based clinical guidelines for the recognition, prevention, and treatment of diabetes and its complications in the wilderness athlete. These clinical practice guidelines define a “wilderness athlete with diabetes” as an individual with type 1 or type 2 diabetes who participates in mild- to vigorous-intensity exercise in a wilderness environment with limited medical access, at altitudes greater than 2500 m (8250 ft), in climatic extremes, and/or with limited access to immediate medical care and supplies.
Methods
Specialists in emergency medicine, primary care, sports medicine, endocrinology, and nutrition convened in the spring of 2018. Relevant articles were identified through the PubMed database using the following keywords: diabetes, insulin, wilderness, expedition, mountaineering, and ultramarathon. The literature search was supplemented by a manual search of articles referenced by articles found in the initial PubMed search. Studies in these categories included randomized controlled trials, observational studies, retrospective studies, case series, and case studies. Abstract-only reports were not included. Conclusions from review articles and recommendations from related practice guidelines were cited to provide background information, but only primary sources were used in the formulation of recommendation grades. When no relevant studies were identified, the panel recommendation was based on perceptions of risk and benefit derived from clinical experience. The panel used a consensus approach to develop recommendations for the wilderness athlete with diabetes, with level of evidence assigned according to the methodology stipulated by the American College of Chest Physicians for grading of evidence and recommendations (see online Supplementary Table). These recommendations are graded on the basis of the quality of supporting evidence and balance between the benefits and risks or burdens for each modality or intervention.
Background
Diabetes mellitus is a metabolic disease characterized by elevated blood glucose resulting from a lack of insulin secretion, reduced insulin sensitivity, or both. Type 2 diabetes is the most common form (90% of individuals with diabetes have type 2 diabetes)15 and is caused by a combination of reduced insulin sensitivity and impaired insulin secretion. Type 2 diabetes has classically been diagnosed in adults, although the rate of diagnosis in adolescents is increasing, and it is often accompanied by hypertension, hyperlipidemia, and obesity. Type 1 diabetes is typically diagnosed in children or young adults and is caused by autoimmune destruction of islet cells in the pancreas, resulting in insulin deficiency. People with type 1 diabetes require treatment with subcutaneous insulin, whereas those with type 2 diabetes may or may not require insulin treatment.
Strenuous exercise and wilderness environments can complicate glycemic control. In managing type 1 and type 2 diabetes, exercise and environmental stressors may also have a complex interaction with commonly used medications, glucose monitoring, and medication delivery. The effects of exercise alone on diabetes have been discussed in detail elsewhere,16,17 so this guide will briefly review physical activity and environmental considerations in the pathophysiology of diabetes.
Glycemic Pathophysiology in Physical Activity
Physical activity in wilderness environments often involves prolonged activity and a combination of aerobic, anaerobic, and high-intensity exercise, so it is important to understand how neurohormonal responses differ with each type of exercise. Different types of physical activity cause distinct metabolic responses in individuals without diabetes. Aerobic activity involves repetitive glucose uptake by large muscle groups. To balance exercise-induced increases in glucose uptake from muscle, insulin secretion decreases. At the same time, counterregulatory hormones, including glucagon cortisol, growth hormone, and catecholamines, are released, promoting endogenous glucose production through glycogenolysis and gluconeogenesis.17, 18, 19 In athletes with diabetes, these counterregulatory hormones may lead to hyperglycemia if adjustments in carbohydrate consumption and insulin dose are not made.
In individuals without diabetes, insulin levels in anaerobic and high-intensity exercise (>80% of ) do not decrease as significantly as in aerobic activities. Rather, catecholamines play a more prominent role in glucose metabolism, although growth hormone and cortisol are important as well.17,20 After anaerobic and high-intensity exercise in individuals without diabetes, insulin levels rise to compensate for the effects of elevations in counterregulatory hormones18,21 (Figure 1).
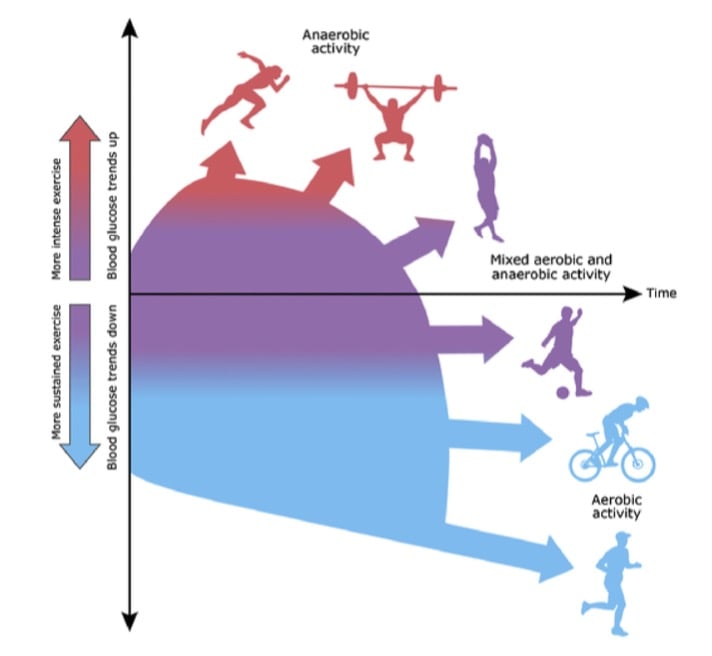
Figure 1. Illustration of different types of exercise including mutual differences in intensities and the way this affects glucose levels. Illustration by Anne Greene, Senior Medical Illustrator, reproduced with permission from UpToDate, Inc. Copyright © 2017 Duration and intensity. Reproduced with permission from: Riddell MC. Management of exercise for children and adolescents with type 1 diabetes mellitus.
After exercise, glucose uptake remains elevated by both insulin-dependent and insulin-independent processes. If exercise is prolonged, insulin-dependent mechanisms can continue up to 48 h after exercise to replenish muscle glycogen stores.16 Exercise lasting greater than 60 min has been shown to increase peripheral insulin sensitivity for up to 48 h post exercise.22, 23, 24, 25 The basic management strategy for type 1 diabetes of decreasing basal insulin rate while increasing carbohydrate intake during exercise to match energy utilization is based on these principles.15,26,27 It is important to understand the increased risk of hypoglycemia that occurs after exercise, as frequent hypoglycemic events in individuals with diabetes have been linked with long-term morbidity and mortality.28 The glycemic response to exercise changes with time of day, and risk for nocturnal hypoglycemia is highest when exercise is completed in the evening.29
In summary, brief high-intensity exercise has been shown to cause hyperglycemia,30 whereas long-duration aerobic exercise is more likely to cause hypoglycemia31 (Figure 1). The glycemic response to exercise is complex and varies from individual to individual.32 For example, muscle damage, such as in an abrupt increase in the level of exercise, may decrease insulin sensitivity.33 Additionally, the effects of various environmental factors on glycemic control will be discussed in detail. Although we attempt to provide evidence-based guidance on insulin management in athletes with diabetes, the care of each individual must be managed thoughtfully.
Pretrip Planning
Preparation is an essential component of any expedition, and even more so for the wilderness athlete with diabetes. Pretrip preparations should generally include appropriate medical screening; attention to physical fitness; planning of insulin regimen, diet, and hydration; and education and discussion on the chosen activity and how it may alter or interfere with an individual’s typical diabetes care. Lastly, it is important to ensure packing of all essential diabetes-specific medical supplies and consideration of contingency and emergency planning.
PREPARTICIPATION MEDICAL EVALUATION AND COUNSELING
The American Diabetes Association does not recommend routine medical clearance for asymptomatic individuals with diabetes before low- to moderate-intensity physical activity.16 However, given the nature of intense physical activity in resource-limited environments, it is prudent for wilderness athletes with diabetes to discuss their plans with their primary care provider and/or endocrinologist prior to travel. Attention to regular follow-up and annual screening tests including ophthalmologic examinations is recommended.
Recommendation
Diabetes-specific healthcare maintenance should be up to date prior to wilderness activity. Athletes with diabetes may need to undergo additional and more frequent specialty evaluations (Evidence grade: 1C).
Athletes with diabetes should meet with their primary care provider and/or endocrinologist prior to wilderness travel (Evidence grade: 1C).
Cardiovascular screening
Individuals with diabetes are at increased risk of ischemic heart disease,34 and individuals with type 1 diabetes have higher rates of silent myocardial ischemia and coronary artery disease than the general population.34 Extrapolation is limited because the majority of studies on those with type 1 diabetes in wilderness settings have excluded any patient with electrocardiogram (ECG) abnormalities.35,36 Some groups have recommended baseline ECG testing prior to wilderness activity,37 and consensus guidelines from the Undersea and Hyperbaric Medical Society and Divers Alert Network have proposed routine preparticipation exercise ECG in adults with diabetes over the age of 40 y prior to scuba diving.38
However, there are differing opinions regarding the utility of ECG or exercise stress testing in asymptomatic individuals to prevent sudden cardiac death during exercise.39, 40, 41, 42 The American College of Sports Medicine recommends a visit with a medical provider for asymptomatic individuals with diabetes prior to vigorous activity. Neither the American Diabetes Association nor the American College of Sports Medicine recommends routine ECG or exercise stress test to screen for cardiovascular disease.41,43 The United States Preventive Services Task Force recommends assessment of 10 y cardiovascular disease risk to guide decisions on the utility of further testing for cardiovascular disease.42 The American Heart Association recognizes possible value of exercise testing in people with diabetes before starting an exercise program, despite conflicting evidence regarding its efficacy for prevention of adverse cardiovascular outcomes.39,40 The need for additional cardiac testing should be based on individual risk factors.39,41,42 Tools such as the atherosclerotic cardiovascular disease Risk Estimator from the American College of Cardiology—which takes into account risk factors such as presence of diabetes, age, and sex—can be useful for cardiovascular risk assessment and shared decision making.44
Recommendations
Individuals with diabetes should undergo comprehensive risk assessment for cardiovascular disease with their primary care provider and/or endocrinologist prior to wilderness travel (Evidence grade: 1B).
Routine pre-participation ECG screening of wilderness athletes with diabetes is not recommended (Evidence grade: 2C).
Routine exercise ECG to screen for coronary artery disease in asymptomatic wilderness athletes with diabetes is not recommended (Evidence grade: 1B).
Diabetes-specific medical conditions
Special attention should be paid to those with diabetes and peripheral neuropathy, nephropathy, or retinopathy because these conditions pose additional risks in wilderness activities.37,45 Those with peripheral neuropathy should be aware of the increased risk of developing cold-induced injury and should be screened for wounds before a wilderness excursion. Foot care should be reviewed prior to activity.37 People with diabetes living at high altitude may have higher rates of albuminuria, though there is no evidence to suggest that short-term altitude exposure is nephrotoxic.37 Individuals with a history of nephropathy should be counseled regarding appropriate medication use—including the renal risk of nonsteroidal anti-inflammatory drug use46,47—and appropriate hydration. Wilderness sports carry a risk of ocular injuries.48 Although corneal injuries and edema are most often reported in the wilderness athlete,49,50 high altitude retinal hemorrhages are also known to occur,51 but there have been no studies on high altitude’s effect on diabetic retinopathy.48,52 Regardless, any retinal damage carries additional importance in individuals with diabetes, and it is recommended that individuals with diabetes have a dilated fundoscopic examination prior to ascent to high altitude.48 To avoid further complications while in the backcountry, it is important to ensure optimal control of diabetes complications prior to high-risk wilderness activities.
Recommendations
Individuals with pre-existing diabetes complications (including nephropathy, peripheral neuropathy, and retinopathy) should be counseled on minimizing additional risks to these organ systems with wilderness activity (Evidence grade: 1C).
All individuals with diabetes planning high altitude travel should be up to date on yearly dilated fundoscopy. If any degree of retinopathy is present, ophthalmologic risks of wilderness travel should be discussed (Evidence grade: 1C).
SUPPLY PREPARATION
Appropriate supplies and medical equipment should be brought on any wilderness activity.15,53 Table 1 lists specific supplies that should be considered essential for wilderness athletes with diabetes.
Table 1. Medical kit preparation
| Category | Supply | Notes |
|---|---|---|
| Glucose monitoring | Glucometer with case | |
| Backup glucometer supplies | Extra meter batteries and/or charging cable | |
| Test strips | Twice as many as anticipated for trip | |
| Lancets | Twice as many as anticipated for trip | |
| CGM and supplies | If using this glucose monitoring method | |
| Treatment | Insulin | Twice as much as anticipated for trip |
| Extra prescription for medications/supplies | ||
| Needles/Syringes | If not using an insulin pump | |
| Pump with case and needles/syringes | If using this method, should bring needles and syringes in case of pump failure | |
| Extra pump supplies | Batteries and/or charging cable, extra infusion sites, extra pump reservoirs | |
| Backup insulin pump supplies | Needles/Syringes in case of pump failure, extra pump batteries and/or charging cable, extra infusion sites, extra pump reservoirs | |
| Alcohol swabs | Twice as many as anticipated for trip | |
| Sharps container | ||
| Emergency supplies | Glucagon emergency kit | |
| Urine ketone strips | Can use as primary method of ketone monitoring, should bring as backup if using serum ketone meter | |
| Ketone meter with case | Extra meter batteries and/or charging cable | |
| Prepackaged snacks | ||
| Other | Letter from physician stating need to carry supplies | |
| Insulin management and emergency plan | Developed in conjunction with endocrinologist | |
| Medical identification card/bracelet with emergency contact information | ||
| Health insurance card | ||
| CGM | continuous glucose monitor. |
Recommendation
Wilderness athletes should be counseled on a complete packing list of routine and emergency diabetes supplies (Evidence grade: 1C).
Wilderness athletes should carry documentation of their medical history, basic diabetes management plan, and basic emergency action plan (Evidence grade: 1C).
GLUCOMETERS
Glucometers can fail in the wilderness owing to extreme environmental conditions, water exposure, or physical damage from high-impact activity. Hypobaric hypoxia at high altitude may alter the reaction used to estimate blood glucose in glucometers that use a glucose oxidase reaction (GOX) or a glucose dehydrogenase reaction (GDH).54,55 GDH and GOX systems have been tested in hypobaric chambers up to 4500 m (14,850 ft), with some tending to overestimate blood glucose (0.8 to 15%)56 and some tending to underestimate blood glucose.57 At 2000 m (6560 ft), GDH systems overestimated blood glucose by 3.5 to 8%, whereas GOX systems showed no significant difference from reference values.54 Although high altitude studies have found statistically significant inaccuracies in blood glucometers, the differences were small enough to be of questionable clinical significance. When tested at high (25°C) and low (8°C) temperatures, GOX and GDH systems performed similarly, with tendencies toward both overestimation and underestimation in some systems, with errors of 5 to 10% or less.56,58
Similar to high altitude, temperature appears to affect glucometer function in a clinically insignificant way, although the errors appear to be brand specific and are unpredictable. There are no studies to define the temperature at which a glucometer will fail despite warming, but caution should be taken and the manual for a device reviewed. In cold environments, glucometers should be kept inside a jacket pocket close to the body during the day and in a sleeping bag at night. Each product guide should be reviewed to determine the altitude level for which a glucometer is approved. Athletes should have at least 1 backup glucometer in case of glucometer failure or loss.
Recommendations
- For glucometers and other monitoring equipment, the product guide should be reviewed carefully before an expedition. Individuals should carry a backup monitor and battery supply (Evidence grade: 1C).
INSULIN Insulin requires safe use and storage in wilderness environments. Each insulin package insert should be read carefully to ensure proper transportation and storage, and insulin should be discarded after an appropriate amount of time.59,60
Insulin is a protein that is denatured by freezing or extreme heat and thus should not be used if exposed to extreme temperatures. Insulin should not be exposed to extreme temperatures (<2 or="">30°C). Likewise, light exposure can potentially reduce insulin’s efficacy. Excess agitation should be avoided because this can lead to clumping and decreased potency.60 In extreme ambient temperatures, measures should be taken to keep insulin at a stable cool temperature.59 This may be accomplished by storing insulin in a well-insulated container (cooler or thermos) with a hard case to prevent vials/pens from breaking. Likewise, using a gel or evaporative cooling pack in warm temperatures can help maintain potency. In hot environments, cooling systems may be used, and the insulin should be stored in the middle of the pack to minimize heat exposure. There are commercially available insulin storage products which use evaporation of cold water to keep vials and pens cold. In cold temperatures, insulin should not only be kept in an insulated container, but should also be carried close to the body during the day and kept inside a sleeping bag at night to prevent freezing. A backup supply of insulin should be kept in an insulated container in the middle of the backpack to prevent exposure to extreme temperatures. It is prudent for any wilderness athlete to have a backup supply of insulin on any expedition. The type of excursion will dictate the safest way to store insulin.
Recommendation
- In the wilderness, insulin should be protected from environmental extremes, such as high or low temperatures, light exposure, and physical agitation. Any method of physical and/or temperature protection should be tested in a low-risk environment prior to use in the wilderness. A contingency supply of insulin should be kept in a separate location (Evidence grade: 1B).
Environmental Considerations
One of the most challenging aspects of diabetes care in the wilderness athlete is to predict and respond to the effects of extreme environmental conditions. The basic effect of different environmental conditions on diabetes is summarized in Table 2.
Table 2. Environmental effects on diabetes
| Environmental factor | Effect on blood glucose | Other effects and risks for wilderness athletes with diabetes |
|---|---|---|
| Extreme altitude (>4000 m) | Increases | Capillary blood glucose testing overestimation |
| Moderate altitude (>1500 – <4000 m) | Unknown | |
| Extreme heat | No direct effect | Increased heat illness, insulin becomes nonviable, increased insulin absorption |
| Extreme cold | Variable | Possible poor insulin absorption, increased cold injuries, insulin freezing |
HIGH ALTITUDE
Change in insulin requirements
High altitude, especially extreme altitude (>5000 m [16,400 ft]), adds greater complexity and difficulty to glucose control in type 1 diabetes. Studies of exercise at sea level showed improved glycemia in individuals with type 1 diabetes who were exposed to a hypoxic environment when compared to a normoxic environment.61 Although there is conflicting evidence on glycemic control at altitudes between 1500 m (4,920 ft) and 4000 m (13,120 ft),36 several studies have observed increased insulin requirements above 4000 m (13,120 ft) in those with type 1 diabetes.35,36 Relative hyperglycemia 62and insulin resistance at extremely high altitudes in people without diabetes have been reported as well.36,63,64 It is unclear whether this observed insulin resistance is a result of acute mountain sickness or exposure to hypobaric hypoxia. Ketosis is an additional risk at high altitude; it is related to suboptimal carbohydrate intake, leading to less insulin administration and higher levels of counterregulatory hormones.35
Recommendation
- Those with insulin-dependent diabetes traveling to high altitude should be counseled on the potential for increased insulin requirements. Athletes should consider close monitoring on shorter trips to learn about their own glycemic trends prior to a major high altitude expedition (Evidence grade: 2C).
High altitude illness
The spectrum of high altitude illnesses includes acute mountain sickness (AMS), high altitude pulmonary edema (HAPE), high altitude cerebral edema (HACE), and high altitude retinal hemorrhage. AMS is defined as a constellation of symptoms including headache, gastrointestinal disturbances, weakness, and dizziness in an individual who has ascended to >2500 m (8,200 ft) and has no other obvious explanation for these symptoms.65 Increased risk of high altitude illness has not been reported in those with diabetes when compared to individuals without diabetes.35,36,66 Although type 1 diabetes itself may not increase the risk of AMS, metabolic decompensation may occur in persons with diabetes who develop AMS, and there have been a few case reports of diabetic ketoacidosis (DKA) in the setting of AMS.67 The symptoms of AMS may confound or mimic symptoms of hypoglycemia or hyperglycemia and make appropriate diagnosis and treatment of type 1 diabetes and ketoacidosis more difficult.37,52 Therefore, if AMS occurs in an individual with diabetes, descent is often the safest option. Although there is limited data on the incidence of HAPE and HACE in those with type 1 diabetes, various case studies have showed no increased incidence for these conditions.35,36,52,68
Recommendations
- Individuals with diabetes should be counseled on symptoms and management options for high altitude illness and dysglycemia. More frequent blood glucose and ketone checks are recommended if symptoms of high altitude illness occur (Evidence grade: 1C).
Acetazolamide
Commonly used in the prophylaxis and treatment of AMS,69,70 acetazolamide is a carbonic anhydrase inhibitor that limits bicarbonate reabsorption in the renal tubules, producing a bicarbonate diuresis and metabolic acidosis.71 Theoretically, this could trigger or worsen acidosis and dehydration and contribute to ketoacidosis.35,68 Although data on the incidence of DKA are inconsistent, there are reports of increased insulin requirements in individuals with type 1 diabetes on acetazolamide, with insulin requirements abruptly returning to normal once acetazolamide was discontinued.67 Also important to consider, paresthesia is a common side effect of acetazolamide and may be confused with hypoglycemic symptoms.35,52
Recommendation
- Acetazolamide should be used with caution in individuals with diabetes (Evidence grade: 2C).
Dexamethasone
Dexamethasone is an oral corticosteroid commonly used in the treatment of AMS, HACE, and HAPE and occasionally is used for prophylaxis of these conditions.69 Dexamethasone is known to cause hyperglycemia and increased insulin requirements.72,73 If considering use of dexamethasone, the risk and severity of high altitude illness needs to be balanced with the risk of and ability to manage resultant hyperglycemia. In particular, it should be noted that in the case of acutely life-threatening conditions such as HACE, the benefits of corticosteroids outweigh the risks of hyperglycemia.
Recommendation
- In wilderness athletes with diabetes, oral corticosteroids should be used with caution in light of the risk of hyperglycemia (Evidence grade: 1C).
COLD ENVIRONMENTS
Extremely cold air temperatures are common in high altitude and other wilderness pursuits and may complicate insulin use in wilderness athletes with diabetes. In individuals without diabetes, metabolic rate increases in response to cold exposure, although no change has been observed in plasma glucose or insulin response to an oral glucose tolerance test.74 Cold acclimation for 10 d has been shown to improve insulin sensitivity in type 2 diabetes.75 However, reduced skin temperatures have been shown to cause decreased insulin absorption within the first 90 min after insulin injection in both type 1 and type 2 diabetes.76 Clinical data are insufficient to accurately predict how cold environments will affect insulin requirements in the field. As discussed, careful consideration needs to be taken with medication storage in cold environments.
Recommendation
- There are insufficient data to describe the effect that cold exposure has on diabetes management (no recommendation).
Cold illness
Individuals with preexisting neuropathy or peripheral vascular disease may be at increased risk for cold-related skin and soft tissue injuries,37,45 and these individuals are at higher risk of frostbite.77
Recommendation
Wilderness athletes with diabetic peripheral neuropathy and peripheral vascular disease are at increased risk of frostbite (Evidence grade: 2C).
HOT ENVIRONMENTS
Extremely hot environments present unique challenges to wilderness athletes with diabetes. In subjects with and without diabetes, higher plasma blood glucose has been observed in response to an oral glucose load during exposure to a hot environment as compared to a neutral or cold environment.78, 79, 80 Extreme heat exposure can lead to increased insulin absorption.81 In the setting of elevated surface temperatures, serum insulin levels were observed to be approximately double for the first 90 min after subcutaneous injection.76 Clinical data are insufficient to accurately predict how hot environments will affect insulin requirements in the field. Insulin itself may denature if exposed to high temperatures (>30°C). As previously discussed, careful consideration needs to be taken with medication storage in hot environments. For expeditions that require repeated bouts of exercise, it is also important to recognize that glycogen resynthesis is impaired in hot environments.82
Recommendation
- There are insufficient data to describe the effect that heat exposure has on diabetes management (no recommendation).
Heat illness
During exercise, cutaneous vascular flow and sweating increase to release excess endogenous heat and maintain core body temperature. Individuals with type 1 diabetes may have impaired sweating during high-intensity exercise.7,83,84 Both type 1 and 2 diabetes may result in impaired exertional heat loss in hot environments.6,7,84 It is theorized that those with diabetes have a blunted sensitivity to elevated ambient temperatures and an inadequate cutaneous vascular perfusion or sweat production response. Age, long-term hyperglycemia, and neuropathy further impair blood flow to the skin, putting individuals with diabetes at higher risk of heat illness.85,86 The ADA recommends that older adults with diabetes avoid exercising outdoors on very hot and/or humid days.16 For relatively young adults, close blood glucose monitoring for hyperglycemia is important during heat exposure to avoid exacerbating potential existing impairments in the body’s cooling mechanisms.
Recommendation
- Wilderness athletes with diabetes are at increased risk for heat illness (Evidence grade: 1C).
MONITORING AND TREATMENT
Glucose monitoring
Prior recommendations on athletic participation for individuals with diabetes have provided reasonable suggestions for blood glucose monitoring and involvement.16,53 Ideally, pre-exercise blood glucose monitoring should be performed 2 to 3 times at 30 min intervals to determine a blood glucose trend; during exercise, every 30 min; and after exercise, every 2 to 4 h owing to potential delayed hypoglycemia.53 It has been suggested that the ideal blood glucose level for exercise is between 130 and 180 mg·dL-1,16,26,53 though it is preferable to have individualized targets based on an athlete’s personal history. Because such time-intensive glucose monitoring is difficult in the wilderness, it is prudent for an individual to establish trends in insulin and carbohydrate requirements during exercise and in similar environmental conditions prior to the expedition. Patients with well-established exercise insulin requirements may check blood glucose prior to exercise and every few hours during prolonged periods of exertion. Continuous glucose monitors (CGM), which will be discussed separately, may be a useful adjunctive tool for close monitoring of blood glucose during wilderness activities, though robust clinical data are lacking.
Recommendation
- In insulin-dependent diabetes, blood glucose should be monitored before, during, and after intense and/or prolonged exercise (Evidence grade: 1B).
Carbohydrate intake
The goal of euglycemia for health and performance in individuals with diabetes requires managing both carbohydrate intake and insulin treatment. Increased carbohydrate intake and/or reduction in insulin dose is typically required to reach the goal of euglycemia during exercise. Past studies suggest 10 to 15 g of carbohydrate is needed to prevent hypoglycemia for low- to moderate-intensity aerobic activity lasting 30 to 60 min.87 A similar amount of activity following an insulin bolus may require 30 to 60 g of carbohydrate to maintain euglycemia.88 A composite of specific guidelines for carbohydrate intake from several organizations are shown in Table 3.16,53,87 Specifically regarding travel in the wilderness, frequent snacks are recommended to maintain energy needs for prolonged physical activity, and we generally recommend increased carbohydrate intake to help mitigate the risk of altitude illness.
Table 3. Blood glucose monitoring and treatment strategy around exercise
| Pre-exercise blood glucose level (mg·dL-1) | Carbohydrate intake recommendation |
|---|---|
| <90 | For typical activities, consume 10–30 g of fast-acting CHO prior to start of exercise |
| For brief or very high-intensity activities, consider no additional CHO intake | |
| For prolonged activities consume an additional 0.5-1.0g per kg body mass per h of exercise based on BG monitoring | |
| Or, delay exercise until blood glucose is >90 mg·dL-1 and monitor closely for hypoglycemia | |
| 90–124 | Consume 10 g of glucose before starting aerobic exercise |
| Can start anaerobic or high-intensity interval exercise right away | |
| 125–150 (alternate 125–180) | Start consuming 0.5-1.0 g per kg body mass per h of exercise at onset of exercise |
| May need to adjust based on type of exercise and insulin | |
| 150–250 (alternate 180–270) | Delay carbohydrate intake until BG levels are below 150 mg·dL-1 |
| Consume non-CHO-containing fluid to prevent dehydration | |
| 250–350 | Test for ketones |
| Delay intense exercise until BG levels are below 250 mg·dL-1 | |
| >350 with no ketones | Consider conservative insulin correction (eg, 50%) before exercise |
| Initiate mild- to moderate-intensity exercise | |
| Delay intense exercise until BG levels are below 250 mg·dL-1 | |
| >250+ ketosis | Avoid exercise if moderate to large amounts of ketones are present |
BG, blood glucose; CHO, carbohydrate.
Guidelines from different groups have recommended different ranges for blood glucose monitoring in exercise.16,53,87
Recommendation
- Those planning protocols for glucose monitoring and carbohydrate intake in exercise should understand how to adjust carbohydrate intake based on blood glucose and exercise. This plan should be individualized based on patients’ medical and exercise history and the environmental stressors to which they are exposed (Evidence grade:1B).
Hydration
Adequate hydration is important before, during, and after exercise. Hyperglycemia may predispose to dehydration and electrolyte (potassium, magnesium, phosphorous) loss through osmotic diuresis. In general, wilderness athletes are able to participate safely if they drink “according to thirst,”89,90 but people with diabetes should hydrate more frequently, especially in the setting of hyperglycemia, ketosis, and illness related to environmental stressors (eg, altitude illness, heat illness).15,91 Specific medications that may affect hydration should be discussed with a medical provider prior to travel. There are no evidence-based protocols for managing hydration in individuals with diabetes during exercise. Similar to insulin and carbohydrate management, it is prudent to base the hydration strategy of an individual with diabetes on experience in similar environmental conditions prior to an expedition.
Recommendation
- Individual hydration strategies should be developed prior to embarking on wilderness activities and should be adjusted based on real-time factors, including environmental temperature, altitude, and exercise type and duration (Evidence grade: 1C).
Insulin management
It has been suggested that individuals using multiple daily injections (MDI) of insulin should have their basal insulin rate reduced by 20% for doses before and after exercise. With continuous subcutaneous insulin infusion (CSII), athletes can reduce or suspend basal insulin infusion at the start of exercise, or even 30 to 60 min before exercise, to prevent hypoglycemia. Reduction in insulin boluses by 25 to 75% 2 to 3 h prior to activity may limit hypoglycemia.16,91 Although guidelines are helpful, it is important to consider the variables of an individual and trip; not everyone will need to decrease their insulin during wilderness pursuits. Owing to factors related to environmental extremes and prolonged physical activity, insulin needs may increase.
Multiple attempts have been made to develop uniform guidelines for glucose monitoring, carbohydrate intake, and insulin dosing during exercise. Generalized management recommendations can be unreliable owing to variable individual glycemic responses to physical activity as well as environmental conditions. Individuals with type 1 diabetes should discuss individual management strategies with their endocrinologist prior to wilderness activities, and an individual management plan should be based on one’s previous glycemic responses to similar exercise and/or environmental conditions. Individuals using an insulin pump should carry a backup method of insulin delivery. Additionally, it is important for wilderness athletes with diabetes to be at an appropriate level of physical fitness and to develop an action plan regarding potential complications prior to an expedition.
Recommendation
- Wilderness athletes with type 1 diabetes should understand how to adjust insulin doses via either MDI or CSII. This should be individualized based on their medical and exercise history and the environment to which they are exposed. This should be discussed in detail with their primary care provider and/or endocrinologist prior to embarking on wilderness activities. Any device should be explained thoroughly prior to an expedition (Evidence grade: 1B).
Noninsulin medications
Although the majority of this clinical practice guideline discusses insulin-treated type 1 and type 2 diabetes, special consideration should be given to common noninsulin medications used in the treatment of diabetes and their unique side effects and potential complications (Table 4).
Table 4. Noninsulin diabetes medications
| Medication | Important side effects | Incidence |
|---|---|---|
| Metformin | Diarrhea | 10–30% |
| Nausea | 10–15% | |
| Lactic acidosis | 2–5/100,000 | |
| Sulfonylureas | Hypoglycemia | Frequency varies by degree of hypoglycemia |
| Glinides | Hypoglycemia | Frequency varies by degree of hypoglycemia |
| Acarbose | Abdominal pain | 19% |
| Diarrhea | 31% | |
| DPP4 inhibitors | Nausea | 10–24% |
| Diarrhea | 10–18% | |
| SGLT2 inhibitors | Diuresis (hypotension, dehydration) | 1% |
| Increased risk of UTIs | <1% (19 cases of urosepsis reported over 1 year) | |
| Euglycemic diabetic ketoacidosis | <1% (73 cases reported over 2 years) | |
| GLP-1 receptor agonists Nausea/Vomiting 10–30% |
Diarrhea 10–15% AP, artificial pancreas; BG, blood glucose; CGM, continuous glucose monitoring; DPP4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide 1; HCL, hybrid closed loop; MDI, multiple daily injection; SGLT2, sodium-glucose transport protein 2; UTI, urinary tract infection.
Metformin is a commonly used medication, prescribed to 84% of people with type 2 diabetes in the United Kingdom.92 Metformin is a biguanide class antihyperglycemic that improves glycemia by reducing hepatic gluconeogenesis and increasing peripheral insulin-mediated glucose utilization. Metformin may cause diarrhea, and 25% experience some form of digestive tract disturbance upon starting the medication. Metformin-induced diarrhea may predispose to dehydration and hypokalemia.93 There is a known risk of metformin-induced lactic acidosis, found at a rate of 2 to 5 per 100,000,94 with elevated serum creatinine being the greatest predisposing risk factor. Although concurrent use of metformin and acetazolamide (a diuretic carbonic anhydrase inhibitor commonly used for AMS prophylaxis) has not been directly assessed, there is concern that concurrent use of a diuretic with metformin could precipitate lactic acidosis.94,95 Exercise alone is known to be safe while using metformin alone,16 and no dose adjustments are recommended.
Both sulfonylurea and glinide class medications carry a risk of hypoglycemia, and blood glucose should be monitored following initiation or changes in these medications. Acarbose and DPP4 inhibitor medications including sitagliptin and saxagliptin can cause vomiting and diarrhea.96,97 Hypoglycemia appears to be a minimal risk with the DPP4 medications.97 SGLT2 inhibitors block glucose reuptake in renal tubules and so have diuretic-like effects that could theoretically worsen dehydration, though there is no supporting clinical evidence.98 SGLT2 inhibitors pose a known increased risk of urinary tract infections and euglycemic DKA, carrying a specific Food and Drug Administration warning.98,99 Glucagon-like peptide-1 receptor agonists are a relatively new class of injectable antihyperglycemic medication, with nausea, vomiting, and diarrhea being the most frequently reported gastrointestinal side effects.100
Noninsulin diabetes medications should ideally be stored in a dark environment at room temperature, although compared with insulin, they are not as sensitive to environmental perturbations.
Recommendation
Use of noninsulin diabetes medications should not be considered a contraindication to wilderness athletic involvement, though participants should be cautious regarding side effects. Particular attention should be paid to the individual risks of each specific class of medication (Evidence grade: 1C).
GLYCEMIC COMPLICATIONS
In addition to proper pre-trip planning and understanding the basics of carbohydrate intake and insulin management, several specific complications and medical issues deserve unique consideration for the wilderness athlete with diabetes.
Hypoglycemia
Hypoglycemia is defined as a plasma glucose level less than 70 mg·dL-1, is a well-recognized hindrance to athletic performance, and most importantly poses an acute health risk in diabetes. Typical symptoms of hypoglycemia include drowsiness, confusion, dizziness, nausea, palpitations, tremor, sweating, and anxiety. As a result of hormonal counterregulatory mechanisms, hypoglycemia is also accompanied by hypokalemia, which can last for several hours after blood glucose levels have returned to normal101 and can cause impaired skeletal muscle contraction, weakness, and potential cardiac arrhythmias.91
The primary treatment option for hypoglycemia is glucose repletion. Although common options typically are oral, rectal, and intravenous (IV) repletion, IV repletion should not be relied on as an option in the wilderness setting. Therefore, it is important to assess the severity of a hypoglycemic episode to decide on the safety and utility of oral treatment or whether evacuation is necessary. The basic principles of hypoglycemia management in the wilderness are cessation of insulin treatment and glucose repletion. Glucose repletion can be accomplished either directly or by administration of glucagon, which stimulates gluconeogenesis and glycogenolysis102 (Table 5).103,104 High glycemic index carbohydrates are best for oral glucose repletion, and16 in the wilderness, low-weight options such as sugar packets, sugar cubes, glucose gel, honey, corn syrup, and glucose tablets can be considered. For severe hypoglycemia—defined as severe cognitive impairment requiring external assistance for recovery—glucagon is the first-line treatment. Any glucagon treatment should be followed by carbohydrate intake to prevent rebound hypoglycemia, especially in the setting of depleted glycogen stores.105,106 Similar to insulin, glucagon should be stored with insulation to avoid extremes of heat and cold. Individuals accompanying those with diabetes should know where glucagon is stored and how and when to administer it. It is important to consider the risk of ketosis if insulin therapy is suspended for more than 2 to 3 h.107
Table 5. Treatment of hypoglycemia in the backcountry
Medication Dose Route Timing Side effects Special considerations Glucose 15–20 g PO, PR, IV Repeat dose q15 min PRN for persistent hypoglycemia Hyperglycemia Patient must immediately consume meal or carbohydrate snack after resolution of hypoglycemia. Glucagon 1 mg IM, “mini-dose” subcutaneous, intranasal Effective in 10–15 min Nausea, vomiting Only if able to take oral glucose. Effectiveness limited in glycogen-depleted patients. IM, intramuscular; IV, intravenous; PO, oral administration; PR, per rectum; PRN, as needed.
Individuals with type 1 diabetes are at risk for delayed nocturnal hypoglycemia following daytime exercise. There are multiple reasons for this phenomenon, including a rise in insulin sensitivity after exercise, increased glucose uptake by skeletal muscles to replenish glucose stores, and impaired counterregulation in response to hypoglycemia.22 For those with MDI, the risk of nocturnal hypoglycemia may be minimized through approximately 20% reduction of daily basal insulin dose, reduced prandial bolus insulin, and low glycemic index carbohydrate feeding after evening exercise. For those using CSII, basal rate reductions of approximately 20% at bedtime for 6 h after afternoon exercise may limit nocturnal hypoglycemia. Other strategies include a bedtime snack, glucose checks overnight, and/or use of CGM with alarms and automatic pump suspension.91
Recommendation Wilderness athletes with diabetes should have a plan and carry supplies for treating hypoglycemia. They should be prepared to use a glucose repletion and glucagon strategy (Evidence grade: 1C).
Wilderness athletes with diabetes should have experience with individualized methods for managing nocturnal hypoglycemia prior to wilderness activity (Evidence grade: 1C).
Hyperglycemia If someone with diabetes is found to be hyperglycemic (plasma glucose level >250 mg·dL-1), it is important to determine whether the individual is in an acute hyperglycemic crisis, including hyperosmolar hyperglycemic state (HHS) or DKA. To help differentiate between hyperglycemia and HHS/DKA, individuals with type 1 diabetes should be able to test for blood or urine ketones if they have unexplained hyperglycemia.
Hyperosmolar hyperglycemic state HHS is a state of progressive hyperglycemia and hyperosmolarity typically seen in individuals with poorly controlled or undiagnosed type 2 diabetes, limited access to water, and a precipitating medical event. The development of HHS is attributed to insulin resistance, deficiency, or both, in addition to increased hepatic gluconeogenesis, osmotic diuresis, and dehydration. The hyperglycemia in HHS is profound, with serum glucose level usually >600 mg·dL-1 and extreme dehydration with a fluid deficit of 8 to 12 L.108
Diabetic ketoacidosis
DKA occurs predominantly in type 1 diabetes and results from absolute insulin deficiency. Alternate fuel stores are broken down, leading to hyperglycemia, ketosis, dehydration, and acidosis. Without insulin to correct the acidosis, DKA ensues. DKA is defined by a blood glucose level >250 mg·dL-1, an anion gap >10, pH <7.3, and="" ketonemia.
Both ketosis and DKA have been reported in the wilderness setting.35,109 DKA and HHS may be precipitated by infections, heat illness, dehydration, cardiac ischemia, major trauma, and medications including steroids and diuretics. Thus, if one of these conditions is diagnosed, it is important to consider the underlying trigger.108 Both DKA and HHS are potentially life-threatening situations, and hospital-based treatment includes IV fluids and insulin, hourly blood tests, electrolyte replacement, close cardiovascular and neurological monitoring, and slow correction of acidosis and dehydration. In the wilderness environment, those interventions are unavailable, and so focus should be on close monitoring and prevention of worsening ketosis. Our panel has experience managing ketosis in the backcountry with oral hydration and subcutaneous insulin. However, given the high mortality potential of both conditions, one should have a low threshold for evacuation if there is clinical suspicion for true HHS or DKA. In the wilderness setting, it is important to prevent potentially severe or life-threatening conditions with careful planning.
Ketone monitoring and management Symptoms of ketosis, including nausea, vomiting, fatigue, tachycardia, and lethargy, can mimic dehydration, gastroenteritis, heat illness, exercise-associated hyponatremia, or altitude illness. Signs and symptoms of ketosis should be reviewed carefully prior to wilderness activities. Individuals with diabetes in the wilderness should have some method for monitoring ketones. Monitoring options for ketones include urine ketone strips and serum ketone monitoring using a fingerstick monitor similar to a blood glucometer. Serum ketone measurement may be preferred over urine ketone testing84 because this method provides real-time levels of beta-hydroxybutyrate in the blood. Urine ketones measure bladder ketone levels, which may reflect ketone levels that were previously higher.108 Both modalities are small, light, and reasonable to carry in a medical kit. Expiration dates for ketone strips should be checked carefully prior to travel because strips may be inaccurate after their use-by date.107 Temperature guidelines for ketone monitors are similar to glucometers, and it is important to verify the temperature and altitude limitation for each type of ketone monitor (ie, some are approved from –25 to 55°C, and up to 7100 m). Ketone monitoring strategies should be discussed with anyone with type 1 or type 2 diabetes with insulin deficiency, type 2 diabetes requiring insulin injections, or someone who has known or suspected ketosis-prone type 2 diabetes.110
Recommendation Those with insulin-dependent diabetes should know the signs and symptoms of ketosis, carry a serum and/or urine ketone testing kit, and know how to treat ketones during wilderness activities. It may be prudent to carry both as a contingency in the event of failure due to environmental conditions (Evidence grade: 1B).
DKA and HHS are medical emergencies, but in the wilderness setting, it may be possible to prevent progression to advanced illness with early recognition of ketosis and treatment with subcutaneous insulin and oral hydration/glucose repletion. Symptoms of ketosis, particularly vomiting or a change in mental status,110 or blood glucose level elevated above 250 mg·dL-1 should prompt ketone monitoring. In general, any ketosis is a sign of insulin deficiency, and in the setting of hyperglycemia, moderate or large amounts of ketones on serum or urine monitoring reflects actual or impending DKA. The general strategy for management of ketosis involves increased insulin availability along with attention to maintaining carbohydrate availability, generous oral hydration, and modification or cessation of physical activity.87 Figure 2 outlines a proposed guideline for treatment of ketosis in the backcountry, with the goal of early recognition and prevention of progression to ketoacidosis and subsequent need for evacuation. Prior to a wilderness expedition, the wilderness athlete should discuss a plan for insulin adjustment, treatment of ketosis, and criteria for evacuation with their endocrinologist.
Figure 2 Download : Download high-res image (1MB)Download : Download full-size image Figure 2. Algorithm for management of hyperglycemia and ketosis in the backcountry. EDD, estimated daily dose; PO, oral intake.
Recommendation Ketosis may be safely managed in the wilderness if an athlete with diabetes and the athlete’s healthcare provider are comfortable with a treatment protocol and if the patient is able to take oral hydration and nutrition and shows no signs of altered mental status (Evidence grade: 2C).
Both HHS and DKA should be considered medical emergencies managed by emergent removal or evacuation to definitive care (Evidence grade: 1A).
Healthcare providers covering events or expeditions in the wilderness should have the ability to monitor blood glucose and ketones and have a basic familiarity with how to treat and triage glucose abnormalities (Evidence grade: 1C).
There should be a plan for evacuation in the case of a hyperglycemic emergency (Evidence grade: 1A).
Exercise-induced hyperglycemia Exercise-induced hyperglycemia is common in type 1 and type 2 diabetes. In wilderness athletes specifically, there seems to be increased insulin requirements at some environmental extremes.35,36 Decreased insulin doses before exercise can promote a rise in blood glucose, as can malfunctioning insulin pump infusion sets. Overconsumption of carbohydrates before or during exercise, along with aggressive insulin reduction, can result in hyperglycemia during any exercise. The risk of hyperglycemia during exercise may be mitigated if intense activities are interspersed between moderate-intensity aerobic activities. Similarly, combining resistance training (first) with aerobic training (second) optimizes glucose stability in those with type 1 diabetes. Options to correct postexercise hyperglycemia include a conservative insulin correction (50% of usual) and/or an aerobic cool down.16 It is important to remember that excessive insulin corrections after exercise may increase the risk of nocturnal hypoglycemia. As previously discussed, attention should also be paid to pre-trip level of fitness to mitigate glycemic complications from exercise.
Recommendation Those with insulin-dependent diabetes should understand how to adjust insulin doses when hyperglycemia occurs during activity. This should be based on their individual experiences during exercise, training, and previous exposures to environmental stressors. This should be discussed in detail with their endocrine provider prior to embarking on a wilderness adventure (Evidence grade: 1B).
EMERGING TECHNOLOGY The management of diabetes is on the brink of large-scale change as new technologies become available. CGM and hybrid closed loop insulin delivery systems have ever-growing evidence favoring their use and the potential to alter the standard of diabetes care. The end goal of these developments is a completely autonomous technological system that functions as normal human pancreatic endocrine cells. These technologies offer the possibility of sophisticated glycemic monitoring and treatment in the wilderness, decreased risk of hypoglycemia, and maintenance of optimal glycemia for performance. Technological innovations may allow access to wilderness pursuits (eg, big wall rock climbing, scuba diving) that have historically been off limits to those with diabetes given excessive risks or difficulty in glycemic monitoring.111 Important considerations for any electronic device include maintaining functionality in extreme environments, limited access to power sources, cleaning, and servicing in case of malfunction.
Multiple studies in individuals with type 1 diabetes comparing either CSII or MDI have validated positive clinical results of CGM, including reduction in HbA1c, reduction in time spent in hypoglycemia, and improvement in quality of life.112, 113, 114, 115 CGM use reduces glycemic anxiety given real-time tracking and hypoglycemia alarm systems, an effect that could be beneficial for the wilderness athlete.116
The US Federal Drug Administration approved a hybrid closed loop insulin delivery system (also known as the artificial pancreas) in October 2016. In this system, a CGM is paired with an insulin pump, and insulin delivery rate is adjusted based on CGM data, directed by an onboard algorithm independent of patient input. Preliminary data shows promise for safety and improved glycemic control.114,117, 118, 119 However, operator input is still required for meal bolus insulin because it remains difficult to autonomously alert an artificial system of an impending meal.
For various reasons, exercise poses a challenge to any automated insulin delivery system.120 To date, only 1 study has evaluated such a system in the wilderness athlete. Those randomized to a single-hormone artificial pancreas versus sensor augmented pump therapy had more time in ideal blood glucose control, reduced time in hypoglycemia, and higher satisfaction with therapy.121 There was no documented device failure for either system in the study’s alpine skiing environment.
A future target of research and development is the addition of other hormones to the artificial pancreas system. Glucagon has been studied most, as diabetes results in impaired secretion.122 One study found improved glycemic control with less time in hypoglycemia with a dual-hormone artificial pancreas, as compared to sensor augmented therapy.123 The future expectation may be that all individuals with diabetes will use CGM, and all insulin-dependent people will be encouraged to use a hybrid closed loop insulin delivery system, which would be a great benefit for the wilderness athlete undergoing high energy expenditure in wilderness environments. Still, resource-limited scenarios with extreme environmental conditions will limit complete technologic dependence on technology, and a backup analog management plan will likely always be necessary (Table 6).
Table 6. Emerging technology
Treatment system Description Pros Cons
4 hormone AP Insulin, glucagon, amylin, C-peptide infusion
•
Complete replacement of hormones lost in type 1 diabetes mellitus
• Theoretical reduction in micro/macrovascular complications
• Only conceptual
• No data to support efficacy of quad hormone replacement
• Not clinically available
Dual hormone AP Pump automatically adjusts basal insulin based on CGM data, user input for meals. Glucagon infusions automatically given for hypoglycemia.
•
Improved control reduced hypoglycemia
• More medication to store/carry/manage
• More complicated software and technology
• Not clinically available
Single hormone AP Pump automatically adjusts basal rate based on CGM data, user input for meals
•
Improved control
• Reduced hypoglycemia
• Requires technological literacy
• Frequent calibration
CGM + insulin pump (“sensor augmented pump”) Pump and CGM do not communicate. Basal rate and meal bolus manually adjusted (some have low glucose suspend feature).
•
If one system fails the other is still functional
• More input required
• Less control compared to AP
CGM + MDI CGM with bolus and basal insulin given as MDI
•
One less device
• Multiple medications needed
• Less control than above
Insulin pump + capillary BG Pump with capillary BG monitoring only
•
No calibration required
• Greater risk of hypoglycemia
MDI + capillary BG MDI for bolus and basal insulin with capillary BG monitoring only
•
No device contained on body
• Lowest level of control and monitoring
AP, artificial pancreas; BG, blood glucose; CGM, continuous glucose monitoring; MDI, multiple daily injection.
Recommendations
- Although there is insufficient in vivo data on continuous glucose monitoring or novel hybrid closed loop insulin delivery systems to recommend their use for wilderness athletes with diabetes, the use of such technology may be considered after discussion with an individual’s endocrine provider (Evidence grade: 1C).
Conclusions
As the number of athletes in wilderness and remote environments increase, the number of wilderness athletes with diabetes will also increase. There is strong evidence that exercise and outdoor activities have health benefits for individuals with diabetes. Individuals with diabetes and the healthcare providers who care for them should be fully equipped to support the pursuit of exercise through wilderness activities. During pre-trip planning, addressing the daily management of diabetes, acute medical issues, and considerations unique to the wilderness environment is a good start; there are so many variables that it is impossible to come up with a single set of guidelines that can be uniformly applied to all wilderness athletes with diabetes.
It is important to tailor and personalize the medical care of each individual based on personal history and input. Attention should be paid to physical preparation for an excursion, including muscular and aerobic fitness, as well as prior exposure to environmental conditions. Insulin doses and diet plans should be adjusted according to the type and degree of activity that will be performed, an individual’s baseline level of fitness, the individual’s athletic and disease history, and the environment to which the athlete will be exposed. Wilderness athletes with diabetes should carry a basic written plan developed with their endocrinologist describing their usual insulin regimen, a plan for basic adjustments in the backcountry, basics of hypo-/hyperglycemia management, and an emergency action plan. It is also important to consider the comfort level of an athlete’s travel companions, available resources, and emergency evacuation options.
We recommend that healthcare professionals and athletes with diabetes approach wilderness activities through shared decision making. When preparing for wilderness pursuits, it may be most important to consider the athlete’s own attitudes toward disease management, awareness of the complexities of their individual disease, and their comfort with daily adjustments. With thoughtful and thorough preparation and planning, the wilderness can be accessible and safe for individuals with diabetes.
Author Contributions: Clinical practice guideline concept and design (KV, LN, RF, CJ, AB, BC, MK, GL); literature review (KV, LN, RF, CJ, AB); drafting of the manuscript (KV, LN, RF, CJ, AB); critical revision of the manuscript (KV, LN, RF, CJ, AB, BC, MK, GL); approval of final manuscript (KV, LN, RF, CJ, AB, BC, MK, GL).
Financial/Material Support: None.
Disclosures: None.
Appendix A. Supplementary materials The following is the Supplementary data to this article: Download : Download Word document (13KB) Supplementary Table 1.
References
Centers for Disease Control and Prevention National Diabetes Statistics Report, 2017 Centers for Disease Control and Prevention, Atlanta, GA (2017)
M.D. Hoffman, E. Krishnan Health and exercise-related medical issues among 1,212 ultramarathon runners: baseline findings from the Ultrarunners Longitudinal TRAcking (ULTRA) Study PLoS One, 9 (1) (2014), Article e83867 CrossRef
M. Khodaee, M. Riederer, K. VanBaak, J.C. Hill Ultraendurance athletes with type 1 diabetes: Leadville 100 experience Wilderness Environ Med, 26 (2) (2015), pp. 273-275 ArticleDownload PDF
P. de Mol, S.T. de Vries, E.J. de Koning, R.O. Gans, H.J. Bilo, C.J. Tack Physical activity at altitude: challenges for people with diabetes: a review Diabetes Care, 37 (8) (2014), pp. 2404-2413
K.J. Nadeau, J.G. Regensteiner, T.A. Bauer, M.S. Brown, J.L. Dorosz, A. Hull, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function J Clin Endocrinol Metab, 95 (2) (2010), pp. 513-521
G.P. Kenny, J.M. Stapleton, J.E. Yardley, P. Boulay, R.J. Sigal Older adults with type 2 diabetes store more heat during exercise Med Sci Sports Exerc, 45 (10) (2013), pp. 1906-1914
M.R. Carter, R. McGinn, J. Barrera-Ramirez, R.J. Sigal, G.P. Kenny Impairments in local heat loss in type 1 diabetes during exercise in the heat Med Sci Sports Exerc, 46 (12) (2014), pp. 2224-2233
D. Patschan, G.A. Muller Acute kidney injury in diabetes mellitus Int J Nephrol, 2016 (2016), p. 6232909
W. Abu-Ashour, L.K. Twells, J.E. Valcour, J.M. Gamble Diabetes and the occurrence of infection in primary care: a matched cohort study BMC Infect Dis, 18 (1) (2018), p. 67
C. Leal, J. Admetlla, G. Viscor, A. Ricart Diabetic retinopathy at high altitude High Alt Med Biol, 9 (1) (2008), pp. 24-27
D. Umpierre, P.A. Ribeiro, C.K. Kramer, C.B. Leitao, A.T. Zucatti, M.J. Azevedo, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis JAMA, 305 (17) (2011), pp. 1790-1799
L. Avery, D. Flynn, A. van Wersch, F.F. Sniehotta, M.I. Trenell Changing physical activity behavior in type 2 diabetes: a systematic review and meta-analysis of behavioral interventions Diabetes Care, 35 (12) (2012), pp. 2681-2689
L. Chen, J.H. Pei, J. Kuang, H.M. Chen, Z. Chen, Z.W. Li, et al. Effect of lifestyle intervention in patients with type 2 diabetes: a meta-analysis Metabolism, 64 (2) (2015), pp. 338-347
D. Sluik, B. Buijsse, R. Muckelbauer, R. Kaaks, B. Teucher, N.F. Johnsen, et al. Physical activity and mortality in individuals with diabetes mellitus: a prospective study and meta-analysis Arch Intern Med, 172 (17) (2012), pp. 1285-1295
G.D. Harris, R.D. White Diabetes in the competitive athlete Curr Sports Med Rep, 11 (6) (2012), pp. 309-315
16 S.R. Colberg, R.J. Sigal, J.E. Yardley, M.C. Riddell, D.W. Dunstan, P.C. Dempsey, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association Diabetes Care, 39 (11) (2016), pp. 2065-2079
17 W. Kindermann, A. Schnabel, W.M. Schmitt, G. Biro, J. Cassens, F. Weber Catecholamines, growth hormone, cortisol, insulin, and sex hormones in anaerobic and aerobic exercise Eur J Appl Physiol Occup Physiol, 49 (3) (1982), pp. 389-399
18 R.C. Camacho, P. Galassetti, S.N. Davis, D.H. Wasserman Glucoregulation during and after exercise in health and insulin-dependent diabetes Exerc Sport Sci Rev, 33 (1) (2005), pp. 17-23
19 P. Galassetti, D. Tate, R.A. Neill, S. Morrey, S.N. Davis Effect of gender on counterregulatory responses to euglycemic exercise in type 1 diabetes J Clin Endocrinol Metab, 87 (11) (2002), pp. 5144-5150
20 A. Eliakim, D. Nemet, G. Most, N. Rakover, M. Pantanowitz, Y. Meckel Effect of gender on the GH-IGF-I response to anaerobic exercise in young adults J Strength Cond Res, 28 (12) (2014), pp. 3411-3415
21 E.B. Marliss, M. Vranic Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes Diabetes, 51 (Suppl 1) (2002), pp. S271-S283
22 M.J. MacDonald Postexercise late-onset hypoglycemia in insulin-dependent diabetic patients Diabetes Care, 10 (5) (1987), pp. 584-588
23 F. Magkos, Y. Tsekouras, S.A. Kavouras, B. Mittendorfer, L.S. Sidossis Improved insulin sensitivity after a single bout of exercise is curvilinearly related to exercise energy expenditure Clin Sci (Lond), 114 (1) (2008), pp. 59-64
24 X. Wang, B.W. Patterson, G.I. Smith, J. Kampelman, D.N. Reeds, S.A. Sullivan, et al. A ∼60-min brisk walk increases insulin-stimulated glucose disposal but has no effect on hepatic and adipose tissue insulin sensitivity in older women J Appl Physiol (1985), 114 (11) (2013), pp. 1563-1568
25 K.J. Mikines, B. Sonne, P.A. Farrell, B. Tronier, H. Galbo Effect of physical exercise on sensitivity and responsiveness to insulin in humans Am J Physiol, 254 (3 Pt 1) (1988), pp. E248-E259
26 R.D. White Insulin pump therapy (continuous subcutaneous insulin infusion) Prim Care, 34 (4) (2007), pp. 845-871 ArticleDownload PDF 27 D.P. Zaharieva, M.C. Riddell Prevention of exercise-associated dysglycemia: a case study-based approach Diabetes Spectr, 28 (1) (2015), pp. 55-62
28 B.M. Frier Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications Nat Rev Endocrinol, 10 (12) (2014), pp. 711-722
29 J.J. Ruegemer, R.W. Squires, H.M. Marsh, M.W. Haymond, P.E. Cryer, R.A. Rizza, et al. Differences between prebreakfast and late afternoon glycemic responses to exercise in IDDM patients Diabetes Care, 13 (2) (1990), pp. 104-110
30 T.H. Mitchell, G. Abraham, A. Schiffrin, L.A. Leiter, E.B. Marliss Hyperglycemia after intense exercise in IDDM subjects during continuous subcutaneous insulin infusion Diabetes Care, 11 (4) (1988), pp. 311-317
31 M.J. Tansey, E. Tsalikian, R.W. Beck, N. Mauras, B.A. Buckingham, S.A. Weinzimer, et al. The effects of aerobic exercise on glucose and counterregulatory hormone concentrations in children with type 1 diabetes Diabetes Care, 29 (1) (2006), pp. 20-25
32 S.A. Biankin, A.B. Jenkins, L.V. Campbell, K.L. Choi, Q.G. Forrest, D.J. Chisholm Target-seeking behavior of plasma glucose with exercise in type 1 diabetes Diabetes Care, 26 (2) (2003), pp. 297-301
33 J.C. Tee, A.N. Bosch, M.I. Lambert Metabolic consequences of exercise-induced muscle damage Sports Med, 37 (10) (2007), pp. 827-836
34 M.C. Brindisi, B. Bouillet, B. Verges, S. Halimi Cardiovascular complications in type 1 diabetes mellitus Diabetes Metab, 36 (5) (2010), pp. 341-344 ArticleDownload PDF 35 K. Moore, N. Vizzard, C. Coleman, J. McMahon, R. Hayes, C.J. Thompson Extreme altitude mountaineering and type 1 diabetes; the Diabetes Federation of Ireland Kilimanjaro Expedition Diabet Med, 18 (9) (2001), pp. 749-755
36 P. Pavan, P. Sarto, L. Merlo, D. Casara, A. Ponchia, R. Biasin, et al. Metabolic and cardiovascular parameters in type 1 diabetes at extreme altitude Med Sci Sports Exerc, 36 (8) (2004), pp. 1283-1289
37 S. Mohajeri, B.A. Perkins, P.L. Brubaker, M.C. Riddell Diabetes, trekking and high altitude: recognizing and preparing for the risks Diabet Med, 32 (11) (2015), pp. 1425-1437
38 N.W. Pollock, D.M. Uguccioni, G. de Lisle Dear Diabetes and recreational diving: guidelines for the future Diving Hyperb Med, 36 (1) (2006), pp. 29-34
39 R.J. Gibbons, G.J. Balady, J.T. Bricker, B.R. Chaitman, G.F. Fletcher, V.F. Froelicher, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation, 106 (14) (2002), pp. 1883-1892
40 M. Lauer, E.S. Froelicher, M. Williams, P. Kligfield Exercise testing in asymptomatic adults: a statement for professionals from the American Heart Association Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention Circulation, 112 (5) (2005), pp. 771-776
41 D. Riebe, B.A. Franklin, P.D. Thompson, C.E. Garber, G.P. Whitfield, M. Magal, et al. Updating ACSM's recommendations for exercise preparticipation health screening Med Sci Sports Exerc, 47 (11) (2015), pp. 2473-2479
42 S.J. Curry, A.H. Krist, D.K. Owens, M.J. Barry, A.B. Caughey, K.W. Davidson, et al. Screening for cardiovascular disease risk with electrocardiography: US Preventive Services Task Force recommendation statement JAMA, 319 (22) (2018), pp. 2308-2314
43
- Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2018 Diabetes Care, 41 (Suppl 1) (2018), pp. S86-S104
44 T.J. Gluckman, R.J. Kovacs, N.J. Stone, D. Damalas, J.B. Mullen, W.J. Oetgen The ASCVD risk estimator app: from concept to the current state J Am Coll Cardiol, 67 (3) (2016), pp. 350-352 ArticleDownload PDF 45 G.P. Kenny, R.J. Sigal, R. McGinn Body temperature regulation in diabetes Temperature (Austin), 3 (1) (2016), pp. 119-145
46 J.R. Bruso, M.D. Hoffman, I.R. Rogers, L. Lee, G. Towle, T. Hew-Butler Rhabdomyolysis and hyponatremia: a cluster of five cases at the 161-km 2009 Western States Endurance Run Wilderness Environ Med, 21 (4) (2010), pp. 303-308 ArticleDownload PDF 47 J. Baker, J.D. Cotter, D.F. Gerrard, M.L. Bell, R.J. Walker Effects of indomethacin and celecoxib on renal function in athletes Med Sci Sports Exerc, 37 (5) (2005), pp. 712-717
48 T.H. Mader, G. Tabin Going to high altitude with preexisting ocular conditions High Alt Med Biol, 4 (4) (2003), pp. 419-430
49 J.A. Ellerton, I. Zuljan, G. Agazzi, J.J. Boyd Eye problems in mountain and remote areas: prevention and onsite treatment--official recommendations of the International Commission for Mountain Emergency Medicine ICAR MEDCOM Wilderness Environ Med, 20 (2) (2009), pp. 169-175 ArticleDownload PDF 50 T.A. Cope, A. Kropelnicki Eye injuries in the extreme environment ultra-marathon runner BMJ Case Rep, 2015 (2015)
51 M.M. Bosch, D. Barthelmes, K. Landau High altitude retinal hemorrhages--an update High Alt Med Biol, 13 (4) (2012), pp. 240-244
52 P.L. Brubaker Adventure travel and type 1 diabetes: the complicating effects of high altitude Diabetes Care, 28 (10) (2005), pp. 2563-2572
53 C.C. Jimenez, M.H. Corcoran, J.T. Crawley, W. Guyton Hornsby, K.S. Peer, R.D. Philbin, et al. National athletic trainers' association position statement: management of the athlete with type 1 diabetes mellitus J Athl Train, 42 (4) (2007), pp. 536-545
54 H. Bilen, A. Kilicaslan, G. Akcay, F. Albayrak Performance of glucose dehydrogenase (GDH) based and glucose oxidase (GOX) based blood glucose meter systems at moderately high altitude J Med Eng Technol, 31 (2) (2007), pp. 152-156
55 B.H. Ginsberg Factors affecting blood glucose monitoring: sources of errors in measurement J Diabetes Sci Technol, 3 (4) (2009), pp. 903-913
56 D. Oberg, C.G. Ostenson Performance of glucose dehydrogenase-and glucose oxidase-based blood glucose meters at high altitude and low temperature Diabetes Care, 28 (5) (2005), p. 1261
57 J.F. Gautier, A.X. Bigard, P. Douce, A. Duvallet, G. Cathelineau Influence of simulated altitude on the performance of five blood glucose meters Diabetes Care, 19 (12) (1996), pp. 1430-1433
58 K. Nerhus, P. Rustad, S. Sandberg Effect of ambient temperature on analytical performance of self-monitoring blood glucose systems Diabetes Technol Ther, 13 (9) (2011), pp. 883-892
59 M.M. Grajower, C.G. Fraser, J.H. Holcombe, M.L. Daugherty, W.C. Harris, M.R. De Felippis, et al. How long should insulin be used once a vial is started? Diabetes Care, 26 (9) (2003), pp. 2665-2666
60 Insulin administration Diabetes Care, 27 (Suppl 1) (2004), pp. S106-S109 61 A. Zebrowska, B. Hall, A. Kochanska-Dziurowicz, G. Janikowska The effect of high intensity physical exercise and hypoxia on glycemia, angiogenic biomarkers and cardiorespiratory function in patients with type 1 diabetes Adv Clin Exp Med, 27 (2) (2018), pp. 207-216
62 N.E. Hill, K. Deighton, J. Matu, S. Misra, N.S. Oliver, C. Newman, et al. Continuous glucose monitoring at high altitude-effects on glucose homeostasis Med Sci Sports Exerc, 50 (8) (2018), pp. 1679-1686
63 G.L. Peltonen, R.L. Scalzo, M.M. Schweder, D.G. Larson, G.J. Luckasen, D. Irwin, et al. Sympathetic inhibition attenuates hypoxia induced insulin resistance in healthy adult humans J Physiol, 590 (11) (2012), pp. 2801-2809
64 J.J. Larsen, J.M. Hansen, N.V. Olsen, H. Galbo, F. Dela The effect of altitude hypoxia on glucose homeostasis in men J Physiol, 504 (Pt 1) (1997), pp. 241-249
65 R.C. Roach, P.H. Hackett, O. Oelz, P. Bartsch, A.M. Luks, M.J. MacInnis, et al. The 2018 Lake Louise Acute Mountain Sickness Score High Alt Med Biol, 19 (1) (2018), pp. 4-6
66 P. de Mol, S.T. de Vries, E.J. de Koning, R.O. Gans, C.J. Tack, H.J. Bilo Increased insulin requirements during exercise at very high altitude in type 1 diabetes Diabetes Care, 34 (3) (2011), pp. 591-595
67 S.C. Miller Diabetic ketoacidosis and acute mountain sickness: case report and review of treatment options in type 1 diabetes mellitus Wilderness Environ Med, 26 (2) (2015), pp. 185-188 ArticleDownload PDF 68 N.S. Kalson, A.J. Davies, S. Stokes, H. Frost, A.G. Whitehead, I. Tyrrell-Marsh, et al. Climbers with diabetes do well on Mount Kilimanjaro Diabet Med, 24 (12) (2007), p. 1496
69 A.M. Luks, S.E. McIntosh, C.K. Grissom, P.S. Auerbach, G.W. Rodway, R.B. Schoene, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of acute altitude illness: 2014 update Wilderness Environ Med, 25 (4 Suppl) (2014), pp. S4-S14 ArticleDownload PDF 70 B. Basnyat, J.H. Gertsch, E.W. Johnson, F. Castro-Marin, Y. Inoue, C. Yeh Efficacy of low-dose acetazolamide (125 mg BID) for the prophylaxis of acute mountain sickness: a prospective, double-blind, randomized, placebo-controlled trial High Alt Med Biol, 4 (1) (2003), pp. 45-52
71 L. Filippi, F. Bagnoli, M. Margollicci, E. Zammarchi, M. Tronchin, F.F. Rubaltelli Pathogenic mechanism, prophylaxis, and therapy of symptomatic acidosis induced by acetazolamide J Investig Med, 50 (2) (2002), pp. 125-132
72 A. Wajngot, A. Giacca, V. Grill, M. Vranic, S. Efendic The diabetogenic effects of glucocorticoids are more pronounced in low- than in high-insulin responders Proc Natl Acad Sci U S A, 89 (13) (1992), pp. 6035-6039
73 J.L. Hwang, R.E. Weiss Steroid-induced diabetes: a clinical and molecular approach to understanding and treatment Diabetes Metab Res Rev, 30 (2) (2014), pp. 96-102
74 A.L. Vallerand, J. Frim, M.F. Kavanagh Plasma glucose and insulin responses to oral and intravenous glucose in cold-exposed humans J Appl Physiol (1985), 65 (6) (1988), pp. 2395-2399
75 M.J. Hanssen, J. Hoeks, B. Brans, A.A. van der Lans, G. Schaart, J.J. van den Driessche, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus Nat Med, 21 (8) (2015), pp. 863-865
76 M. Berger, H.J. Cuppers, H. Hegner, V. Jorgens, P. Berchtold Absorption kinetics and biologic effects of subcutaneously injected insulin preparations Diabetes Care, 5 (2) (1982), pp. 77-91
77 I. Harirchi, A. Arvin, J.H. Vash, V. Zafarmand Frostbite: incidence and predisposing factors in mountaineers Br J Sports Med, 39 (12) (2005), pp. 898-901
78 C.L. Dumke, D.R. Slivka, J.S. Cuddy, W.S. Hailes, S.M. Rose, B.C. Ruby The effect of environmental temperature on glucose and insulin after an oral glucose tolerance test in healthy young men Wilderness Environ Med, 26 (3) (2015), pp. 335-342 ArticleDownload PDF 79 R.G. Moses, M.J. Patterson, J.M. Regan, R. Chaunchaiyakul, N.A. Taylor, A.B. Jenkins A non-linear effect of ambient temperature on apparent glucose tolerance Diabetes Res Clin Pract, 36 (1) (1997), pp. 35-40 ArticleDownload PDF 80 A.O. Akanji, R.N. Oputa The effect of ambient temperature on glucose tolerance Diabet Med, 8 (10) (1991), pp. 946-948
81 V.A. Koivisto Sauna-induced acceleration in insulin absorption from subcutaneous injection site Br Med J, 280 (6229) (1980), pp. 1411-1413
82 M. Naperalsky, B. Ruby, D. Slivka Environmental temperature and glycogen resynthesis Int J Sports Med, 31 (8) (2010), pp. 561-566
83 J.M. Stapleton, J.E. Yardley, P. Boulay, R.J. Sigal, G.P. Kenny Whole-body heat loss during exercise in the heat is not impaired in type 1 diabetes Med Sci Sports Exerc, 45 (9) (2013), pp. 1656-1664
84 L.M. Laffel, C. Limbert, H. Phelan, A. Virmani, J. Wood, S.E. Hofer ISPAD Clinical Practice Consensus Guidelines 2018: sick day management in children and adolescents with diabetes Pediatr Diabetes, 19 (Suppl 27) (2018), pp. 193-204
85 J.E. Yardley, J.M. Stapleton, R.J. Sigal, G.P. Kenny Do heat events pose a greater health risk for individuals with type 2 diabetes? Diabetes Technol Ther, 15 (6) (2013), pp. 520-529
86 J.E. Yardley, J.M. Stapleton, M.R. Carter, R.J. Sigal, G.P. Kenny Is whole-body thermoregulatory function impaired in type 1 diabetes mellitus? Curr Diabetes Rev, 9 (2) (2013), pp. 126-136
87 M.C. Riddell, I.W. Gallen, C.E. Smart, C.E. Taplin, P. Adolfsson, A.N. Lumb, et al. Exercise management in type 1 diabetes: a consensus statement Lancet Diabetes Endocrinol, 5 (5) (2017), pp. 377-390 ArticleDownload PDF 88 M.P. Francescato, G. Stel, E. Stenner, M. Geat Prolonged exercise in type 1 diabetes: performance of a customizable algorithm to estimate the carbohydrate supplements to minimize glycemic imbalances PLoS One, 10 (4) (2015), Article e0125220 CrossRef 89 B.L. Bennett, T. Hew-Butler, M.D. Hoffman, I.R. Rogers, M.H. Rosner Wilderness Medical Society practice guidelines for treatment of exercise-associated hyponatremia: 2014 update Wilderness Environ Med, 25 (4 Suppl) (2014), pp. S30-S42 ArticleDownload PDF 90 M.D. Hoffman, T. Hew-Butler, K.J. Stuempfle Exercise-associated hyponatremia and hydration status in 161-km ultramarathoners Med Sci Sports Exerc, 45 (4) (2013), pp. 784-791
91 J.E. Yardley, S.R. Colberg Update on management of type 1 diabetes and type 2 diabetes in athletes Curr Sports Med Rep, 16 (1) (2017), pp. 38-44
92 I. Pernicova, M. Korbonits Metformin--mode of action and clinical implications for diabetes and cancer Nat Rev Endocrinol, 10 (3) (2014), pp. 143-156
93 A.J. Garber, T.G. Duncan, A.M. Goodman, D.J. Mills, J.L. Rohlf Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial Am J Med, 103 (6) (1997), pp. 491-497 ArticleDownload PDF 94 F.J. Pasquel, R. Klein, A. Adigweme, Z. Hinedi, R. Coralli, J.L. Pimentel, et al. Metformin-associated lactic acidosis Am J Med Sci, 349 (3) (2015), pp. 263-267 ArticleDownload PDF 95 C.T. Supuran Drug interaction considerations in the therapeutic use of carbonic anhydrase inhibitors Expert Opin Drug Metab Toxicol, 12 (4) (2016), pp. 423-431
96 G. Derosa, P. Maffioli Efficacy and safety profile evaluation of acarbose alone and in association with other antidiabetic drugs: a systematic review Clin Ther, 34 (6) (2012), pp. 1221-1236 ArticleDownload PDF 97 S. Subbarayan, M. Kipnes Sitagliptin: a review Expert Opin Pharmacother, 12 (10) (2011), pp. 1613-1622
98 A.J. Scheen SGLT2 inhibitors: benefit/risk balance Curr Diab Rep, 16 (10) (2016), p. 92
99 US Food & Drug Administration FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections Drug Safety Communications (2015)
100 K. Bettge, M. Kahle, M.S. Abd El Aziz, J.J. Meier, M.A. Nauck Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials Diabetes Obes Metab, 19 (3) (2017), pp. 336-347
101 A. Caduff, H.U. Lutz, L. Heinemann, G. Di Benedetto, M.S. Talary, S. Theander Dynamics of blood electrolytes in repeated hyper- and/or hypoglycaemic events in patients with type 1 diabetes Diabetologia, 54 (10) (2011), pp. 2678-2689
102 Glucagon rDNA origin (GlucaGen) and recombinant LH. (no. 12 in a series of articles to promote a better understanding of the use of genetic engineering) J Biotechnol, 79 (2) (2000), pp. 185-189
103 M.W. Haymond, M.J. Redondo, S. McKay, M.J. Cummins, B. Newswanger, J. Kinzell, et al. Nonaqueous, mini-dose glucagon for treatment of mild hypoglycemia in adults with type 1 diabetes: a dose-seeking study Diabetes Care, 39 (3) (2016), pp. 465-468
104 M.R. Rickels, K.J. Ruedy, N.C. Foster, C.A. Piche, H. Dulude, J.L. Sherr, et al. Intranasal glucagon for treatment of insulin-induced hypoglycemia in adults with type 1 diabetes: a randomized crossover noninferiority study Diabetes Care, 39 (2) (2016), pp. 264-270
105 N. Kedia Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach Diabetes Metab Syndr Obes, 4 (2011), pp. 337-346
106 G. Harrism, A. Diment, M. Sulway, M. Wilkinson Glucagon administration – underevaluated and undertaught Practl Diabetes Intern, 18 (1) (2001), pp. 22-25 CrossRef 107 National Collaborating Centre for Women’s and Children’s Health (UK) Management of type 1 diabetes - targets for and monitoring of glycaemic control Diabetes (Type 1 and Type 2) in Children and Young People: Diagnosis and Management, Vol 18, NICE Guideline (2015)
108 D.M. Cline, MJ, R.K. Cydulka, G.D. Meckler, D.A. Handel, S.H. Thomas Tintinalli's Emergency Medicine Manual (7th ed.), McGraw-Hill Companies, Inc. (2012)
109 B. Matejko, A. Gawrecki, M. Wrobel, J. Hohendorff, T. Benbenek-Klupa, M.T. Malecki, et al. Type 1 diabetes at high altitude: performance of personal insulin pumps and patient metabolic control Diabetes Technol Ther, 19 (10) (2017), pp. 600-602
110
- Glycemic Targets: Standards of medical care in diabetes-2018 Diabetes Care, 41 (Suppl 1) (2018), pp. S55-S64
111 R.M. Bergenstal, W.V. Tamborlane, A. Ahmann, J.B. Buse, G. Dailey, S.N. Davis, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes N Engl J Med, 363 (4) (2010), pp. 311-320
112 R.W. Beck, T. Riddlesworth, K. Ruedy, A. Ahmann, R. Bergenstal, S. Haller, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial JAMA, 317 (4) (2017), pp. 371-378
113 M. Lind, W. Polonsky, I.B. Hirsch, T. Heise, J. Bolinder, S. Dahlqvist, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial JAMA, 317 (4) (2017), pp. 379-387
114 T. Battelino, I. Conget, B. Olsen, I. Schutz-Fuhrmann, E. Hommel, R. Hoogma, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial Diabetologia, 55 (12) (2012), pp. 3155-3162
115 W.V. Tamborlane, R.W. Beck, B.W. Bode, B. Buckingham, H.P. Chase, R. Clemons, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes N Engl J Med, 359 (14) (2008), pp. 1464-1476
116 J.J. Chamberlain, D. Dopita, E. Gilgen, A. Neuman Impact of frequent and persistent use of continuous glucose monitoring (CGM) on hypoglycemia fear, frequency of emergency medical treatment, and SMBG frequency after one year J Diabetes Sci Technol, 10 (2) (2015), pp. 383-388
117 J. Soupal, L. Petruzelkova, M. Flekac, T. Pelcl, M. Matoulek, M. Dankova, et al. Comparison of different treatment modalities for type 1 diabetes, including sensor-augmented insulin regimens, in 52 weeks of follow-up: a COMISAIR study Diabetes Technol Ther, 18 (9) (2016), pp. 532-538
118 B.A. Buckingham, G.P. Forlenza, J.E. Pinsker, M.P. Christiansen, R.P. Wadwa, J. Schneider, et al. Safety and feasibility of the OmniPod hybrid closed-loop system in adult, adolescent, and pediatric patients with type 1 diabetes using a personalized model predictive control algorithm Diabetes Technol Ther, 20 (4) (2018), pp. 257-262
119 E. Bekiari, K. Kitsios, H. Thabit, M. Tauschmann, E. Athanasiadou, T. Karagiannis, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis BMJ, 361 (2018), p. k1310 CrossRef 120 M.C. Riddell, D.P. Zaharieva, L. Yavelberg, A. Cinar, V.K. Jamnik Exercise and the development of the artificial pancreas: one of the more difficult series of hurdles J Diabetes Sci Technol, 9 (6) (2015), pp. 1217-1226
121 M.D. Breton, D.R. Chernavvsky, G.P. Forlenza, M.D. DeBoer, J. Robic, R.P. Wadwa, et al. Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study Diabetes Care, 40 (12) (2017), pp. 1644-1650
122 A. Siafarikas, R.J. Johnston, M.K. Bulsara, P. O'Leary, T.W. Jones, E.A. Davis Early loss of the glucagon response to hypoglycemia in adolescents with type 1 diabetes Diabetes Care, 35 (8) (2012), pp. 1757-1762
123 P.G. Jacobs, J. El Youssef, R. Reddy, N. Resalat, D. Branigan, J. Condon, et al. Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy Diabetes Obes Metab, 18 (11) (2016), pp. 1110-1119